
Rapid detection of sentinel lymph node metastases in different techniques and comparison in low-grade breast carcinomas
Detección rápida de metástasis en ganglio centinela y comparación de diferentes técnicas en carcinomas de mama de bajo grado
Sua,Luz Fa;
Silva,Nhora M.a*;
Vidaurreta,Martha b; de la Orden,Virginiab;Veganzones,Silviab; Rafael,Sara b; Maestro de las Casas, María L.b
aClinical Laboratory and Pathology, Fundación Clínica Valle del Lili. Cali, Colombia. e-mail: lufer24@hotmail.com nsilva@fcvl.org
bSection of Genetics, Clinical Analysis, Hospital Clínico San Carlos de Madrid, Madrid, Spain. e-mail: mmaestro.hcsc@salud.madrid.org
Received for publication December 9, 2010 Accepted for publication July 13, 2011
Summary
Introduction: The role of sentinel node biopsy has revolutionized breast cancer treatment. This determination reduces the mobility of a complete axillary lymphadenectomy. The aim of our study is to analyze the value of sentinel node in low-grade histological breast tumors, studied with hematoxylin and eosin techniques, Immunohistochemistry, and molecular chain reaction in real-time quantitative polymerase (RT-PCR).
Materials and methods: In a pilot study we studied a total of 21 patients with histological diagnosis of mucinous carcinoma, adenoid cystic carcinoma, and medullar carcinoma that underwent the sentinel node technique. Once the lymph node was removed, it was sent to pathology, where it was fragmented and evaluated, using between 25% and 50% of the lymph node for molecular biology laboratory studies.
Results: The sentinel nodes studied were 32, corresponding to the 21 patients. Of the 32 lymph nodes analyzed, 29 (90.6%) were negative on histopathological examination and the molecular identification, 2 (6.2%) were positive in both techniques and 1 (3.125%) lymph node was positive with quantitative RT-PCR and negative in histology (H&E), which - subsequently by immunohistochemistry (IHC) - was diagnosed as isolated tumor cells (ITC).
Conclusion: When comparing the techniques of hematoxylin and eosin, immunohistochemistry, and molecular RT-PCR technique, we found greater sensitivity of molecular techniques; this can reduce the false negative and improve diagnosis of sentinel node metastases. Patients with low histological grade carcinomas have high survival rates, less aggressive tumor behavior, and reduced lymph node at diagnosis.
Keywords: Lymphatic metastasis; Breast neoplasm; Polymerase chain reaction; Biopsy; Immunohistochemistry; Lymph node excision.
Resumen
Introducción: El rol de la biopsia del ganglio centinela ha revolucionado el tratamiento del cáncer de mama. Tomar esta determinación disminuye la morbilidad de una linfadenectomía axilar completa. El objetivo del estudio es analizar el valor del ganglio centinela en los tumores de mama de tipo histológico de bajo grado y estudiarlos con las técnicas de hematoxilina y eosina, inmunohistoquímica y molecular de reacción en cadena de la polimerasa en tiempo real cuantitativa (RT-PCR).
Material y métodos: En un estudio piloto se estudiaron 21 pacientes con diagnóstico histológico de carcinoma mucinoso, metaplásicos o adenoide quístico a quienes se les realizó la técnica del ganglio centinela. Una vez extraído el ganglio se envió a anatomía patológica, donde se fragmentó y evaluó. Para el estudio en el laboratorio de biología molecular se utilizó entre 25% y 50% del ganglio.
Resultados: Se estudiaron 32 ganglios centinelas, correspondientes a 21 pacientes; 29 (90.6%) fueron negativos en el examen histopatológico y en la determinación molecular, 2 (6.3%) positivos en ambas técnicas y en 1 (3.1%) hubo discrepancia en el diagnóstico, éste fue positivo con la RT-PCR cuantitativa y negativo en el estudio histológico (H&E), el cual, por inmunohistoquímica (IHC), se diagnosticó como células tumorales aisladas (CTA).
Conclusión: Cuando se compararon las técnicas de hematoxilina y eosina, inmunohistoquímica y la técnica molecular RT-PCR cuantitativa, se comprobó mayor sensibilidad en la técnica molecular, lo que permite disminuir los falsos negativos y tener un mejor diagnóstico de las metástasis en los ganglios centinelas. Las pacientes con carcinomas de tipo histológico de bajo grado tienen una alta sobrevida, un comportamiento tumoral menos agresivo y menor compromiso ganglionar en el momento del diagnóstico.
Palabra clave: Metástasis linfática; Neoplasias de la mama; Reacción en cadena de la polimerasa; Biopsia; Inmunohistoquímica; Excisión del ganglio linfático.
Prior to deploying the surgical technique of the sentinel node, breast cancer treatment included complete axillary dissection for determination of axillary metastases. Currently, the lymph node metastases are the most important predictive factor and studies on the sentinel node technique have facilitated advancing in its detection and conducting adequate locoregional controll.
The anatomopathological study of the lymph nodes posterior to a complete axillary dissection, where two or three sections were made of each node and these were stained with hematoxylin and eosin (H&E), was a routine procedure that did not permit the exhaustive study of all the nodes, nor did it direct diagnostic efforts to one or two of them specifically2.
The sentinel node is defined as the first drainage node of the breast, which is detected via nuclear medicine techniques and/or dye. This determination has diminished the morbidity of a complete axillary lymphadenectomy and has revolutionized breast cancer treatment during the last decade2. It was also established which were the candidate patients for the technique, women with diagnosis of breast cancer in stages I and II, with tumors smaller than 3 cm and without axillary compromise on clinical exam. The study of the sentinel node has diminished lymphadenectomies by 60% at these stages 3.
Intraoperative exploration of the sentinel node with routine techniques (H&E) was presented as a result of continuous development of the analysis of the sentinel node and it was designed to increase benefits for patients and turned surgery into a real possibility. These intraoperative methods, like imprint cytology or frozen biopsy, depend on the expertise of the pathologist and are not standardized, which produces a considerable number of false negatives, so that a significant number of micrometastases are also detected later through postoperative diagnostic methods like immunohistochemistry (IHC)4.
Patients with negative diagnosis by frozen biopsy with posterior positive result through immunohistochemistry methods needed to be subjected to surgical re-interventions that could have been avoided. With the molecular diagnostic analysis (real time PCR multiplex) a new diagnostic dimension has been created. Unlike conventional imprint cytology and frozen section or immunohistochemistry methods, the molecular method admits complete lymph node analysis and, consequently, permits making a final decision within the time frame of surgical intervention.
The rates of false negatives vary from 5% to 45% with hematoxylin and eosin. Consequently, a significant number of patients must be subjected to resurgical intervention. Instead, evaluations with the molecular method have shown specificity and sensitivity above 96% compared to exhaustive postoperative analyses of alternate sections of lymph node tissue5,6.
This study sought to compare the molecular technique (real-time PCR multiplex) with hematoxylin and eosin and immunohistochemistry in detecting metastasis in the sentinel node in low-grade tumors.
Patients and methods
A prospective study and prognosis from November 2004 to May 2010 was conducted at the Hospital Clínico San Carlos de Madrid, in Madrid and approved by the institutional ethics committee.
The total population studied was made up of 348 patients with diagnosis of invasive or micro-invasive breast cancer, candidates for the sentinel node technique, of which 21 fulfilled the inclusion criteria: having a histological diagnosis of low-grade carcinoma according to WHO criteria in stages I and II (smaller than 3 cm), not presenting clinical axillary compromise, satisfactory migration of the radioisotope, interoperative imprint biopsy and frozen biopsy with H&E, and authorized informed consent. The study did not include patients with morbid obesity, prior radiotherapy, unsatisfactory migration of the radioisotope, with over five sentinel nodes, or those with mixed tumors.
Method. Fragments of the sentinel node selected for RT-PCR could not have been used in the frozen biopsy; this lymph node was randomly distributed according to size, 50% to 75% for pathological anatomy, and between 25% and 50% for the molecular technique, transported to the molecular biology laboratory in guanidine solution (RNA STAT-60).
Molecular biology laboratory. The technique used determines Mammaglobin A and CK-19 via RT-PCR through GeneSearch TM Breast Lymph Node; a quantitative test was performed for rapid detection of clinically relevant metastases (>0.2 mm) in the lymph node tissue removed and analyzed in the Smart Cycler equipment. This method was designed to make rapid amplification of nucleic acids and uses mRNA from the genes to be studied. With this equipment, the determination was conducted in 20 minutes.
Quantitative RT-PCR was carried out with a homogenous sample of fresh lymph node tissue; three gene markers were included - Mammaglobin A, CK-19 and an internal control gene, porphobilinogen deaminase (PBGD), and the «housekeeping gene» found in all genes.
The quantitative RT-PCR technique followed these steps: degradation of the lymph node tissue, homogenization and purification of RNA, and then retrotranscription (RNA to DNA) was carried out with GeneSearchTM Breast Lymph Node equipment and DNA amplification (real time PCR multiplex) in Smart Cycler equipment (Figure 1). Therein, the interpretation of the results was carried out according to threshold values, thus: Mammaglobin A <31, CK 19 <30, PBGD <36 (Figures 2 and 3).

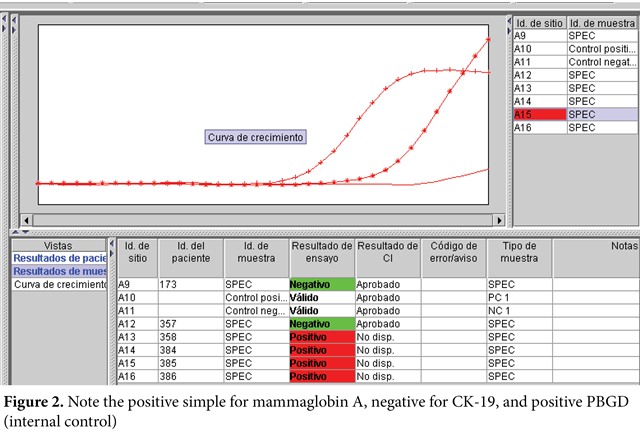
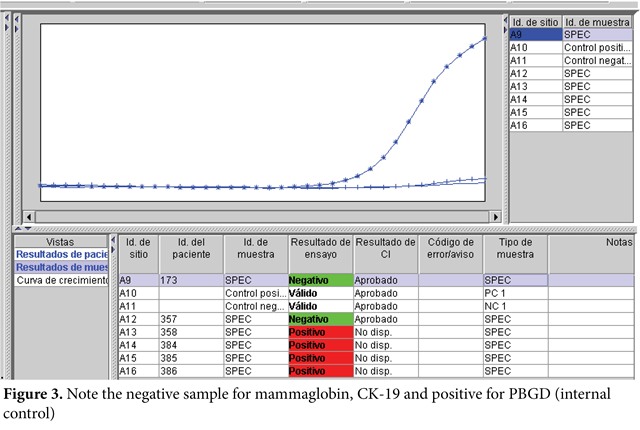
Internal control. Detects mRNA from a gene expressed constitutively in PBGD lymph nodes; such must always be positive to avoid false negatives.
External control. The positive control only has Mammaglobin A and CK19 cancer markers; the negative control only contains PBGD, which must be included in each of the amplifications.
The value of the control is determined when the fluorescent signal surpasses a defined threshold. If the external controls are valid, then the genetic value of the sample is compared to the threshold values of the controls that are specific of each marker.
Interpretation
- If the Ct value of one or both genetic markers is inferior to the threshold value, the sample is determined positive.
- If the Ct value of both genetic markers is superior to the threshold value and the internal control is inferior to the threshold value, the sample is determined negative.
- If the Ct value of the internal control is superior to the threshold value, the simple is determined not valid.
- If the external controls are not valid, it is determined that the whole amplification process is not valid; all the samples of that run must be repeated.
Results
The mean age of the patients was 63 years, with a minimum of 41 years and a maximum of 85 years. The histological type most often found during a 67 month period was mucinous carcinoma in 11 cases, medullar carcinoma in nine, and one case with diagnosis of adenoid cystic carcinoma.
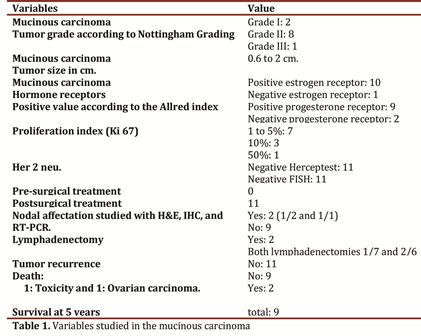
Of the 11 cases of mucinous carcinoma found, eight were tumor grade II, two cases grade I, and one case grade III, with tumor size ranging between 0.6 and 2 cm. The Immunohistochemical study of the hormone receptors corresponds to 10 cases positive estrogen and 1 negative case, 9 cases positive progesterone, and 2 negative cases. The proliferation index studied with Ki-67 in seven cases was less than 5%; three cases at 10% and only one case at 50%. In the 11 cases the Her2 neu oncogene was negative. None of the 11 cases has had tumor recurrence, with survival at five years of nine patients and two deaths by another cause different from breast tumor. The molecular study was coincidentally the quantitative RT-PCR and with IHC in two cases of micrometastases (Table 1).
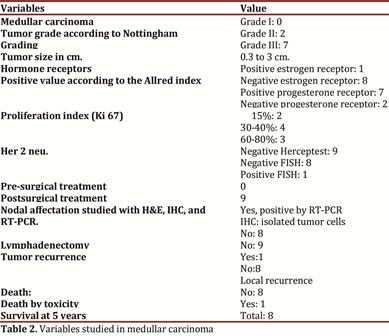
Of the nine medullar carcinomas found, seven had tumor grade III, two cases were grade II, with tumor size ranging between 0.3 and 3 cm. The Immunohistochemical study of the hormone receptors corresponds to one case of positive estrogen and eight negative cases, seven cases positive progesterone and two negative cases. The proliferation index studied via Ki-67 in two cases was at 15%, in four cases at 30 and 40%, and three 3 cases were at 60 and 80%. The Her2 neu oncogene was negative in eight cases and one case was confirmed positive with Fluorescent In Situ Hybridization (FISH). One case showed local tumor recurrence where the molecular study was positive via quantitative RT-PCR and via IHC this case was diagnosed as isolated tumor cells (Table 2), one patient died of causes different from breast tumor, with survival at 5 years of the remaining eight patients.
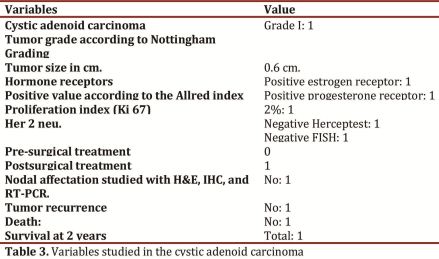
Only one case involved cystic adenoid type breast carcinoma with grade-I tumor, size at 0.6 cm, with positive hormone receptors, proliferation index of 2%, with negative Her2 neu, without tumor recurrence and alive after two years of follow up (Table 3).
In low-grade carcinomas, negativity of the sentinel node was observed at 85.7% (18 cases) and positivity at 14.2% (three cases). Survival at five years is of 17 patients, corresponding to 80.9% and one patient remains alive after two years of follow up.
Three patients died of causes different from breast cancer. Only one patient endured tumor recurrence after four years of follow up, which corresponds to 4.7% of this group of patients.
Of the 32 lymph nodes analyzed, 29 (90.6%) were negative on histopathological exam and on molecular determination; two (6.2%) were positive in both techniques and diagnostic discrepancy was noted in one (3.1%), which was positive with RT-PCR and negative in the histological study, which was thereafter - via IHC - diagnosed as isolated tumor cells.
Discussion
Medullar mammary, mucinous, and cystic adenoid carcinomas are low grade. It is uncommon to find in these axillary metastases and they usually have good prognosis. It was, thus, noted in this series where of the 32 nodes analyzed, 90.6% were negative through
the three techniques evaluated: quantitative RT-PCR, H&E, and IHC. This is reflected on the high free survival of the disease and on the low mortality caused by the tumor. In this article, the rate of false negatives by using H&E, IHC, and quantitative RTPCR is zero due to the increased sensitivity through the molecular technique. On a review of 11 studies, the rate of false negatives fluctuated between 0% and 33%7,8.
The number of positive axillary lymph nodes is important in infiltrating breast cancer. Survival, recurrence, speed of recurrence appearance, and treatment failure are related to the number of positive axillary lymph nodes. Without evidence of lymph node metastasis, survival at five years is at 87%, while the presence of some positive node diminishes it to 75%; by having one to three lymph nodes involved, survival is at 64.5% and with 4 or more positive lymph nodes, survival falls to 34.5%9. In this series, two cases (6.3%) were positive through the three techniques evaluated (quantitative RT-PCR, H&E, and IHC) and one case was positive through quantitative RT-PCR and negative through H&E. Thus, the sensitivity of this molecular technique also agrees with that of IHC. Upon studying the discordant case with IHC, the presence of isolated tumor cells (ITC) was proved, which shows high precision in detecting tumor mRNA in quantitative RT-PCR. On follow up of this patient, tumor recurrence was noted after four years of surgery, which suggests that this condition (ITC) is a negative predicting factor in tumor evolution, but more studies are rendered to clarify the management of these patients.
It has been published that patients with micrometastases studied in serial microscopic sections, and which are smaller than 2 mm, have a survival comparable to patients without lymph node metastasis10.
A prospective study by the international breast cancer study group in Milan, studied the ipsilateral axillary nodes considered negative. Serial sections were made and after the systematic histological study, micrometastases were found in a range from 9% to 20%; these patients with micrometastases had less time of survival free of the disease and global survival of the disease after five years than those whose nodes remained negative after studying them via serial sections11.
The Immunohisto chemical and molecular methods for the diagnosis of metastases hidden in the axillary lymph nodes reveal that patients have a higher recurrence index and worst survival rates especially in high-grade carcinomas12. There were only three such cases in the current series.
The macroscopic size of the infiltrating primary malignant neoplasms is also a relevant prognostic factor. There is direct relationship between tumor size and axillary lymph node metastases. In tumors smaller than 1 cm, there is metastasis at 26%, if the tumor is bigger than 10 cm, there is metastasis at 78%. A tumor with maximum 2 cm diameter has better prognosis and survival rate, as long as it is compared to bigger tumors13. In this series, tumor size is not greater than 3 cm; hence, the lymph node compromise found is only at 3.2%.
Since the work by Mason et al.14 in 1983, which determined the expression of progesterone (PR) and estrogen (ER) of all incidents of breast cancer between 1976 and 1980 in the city of Auckland (1,136 cases) the clinical importance of the positivity for the expression of ER and PR is accepted. In this study it was seen that when both receptors were positive, survival was greater than when they were negative; said effect was proven in the absence of hormone treatments now available to block the replication activity induced by female steroids15. Nowadays, it is accepted as a molecular prognostic factor in localized disease. In our series, in the three tumor types, the proportion of positivity for estrogen and progesterone is predominant.
Regarding the HER-2 neu oncogene, such is expressed at low concentrations in diverse normal cells and are over-expressed in various types of cancer. In general, this fact is caused by genetic amplification or by an increase in the number of erbB-2 genes in the nucleus. This over-expression plays a role in tumor progression; elevated titles are produced of cell growth and oncogenic transformation.
From the practical point of view, the significance of the HER-2 neu oncogene in breast cancer is multiple: breast cancer evolves from the non-invasive premalignant lesion (in situ carcinoma) to the metastatic carcinoma and passes the localized stages. It is possible to find over-expression of HER-2 neu in all stages of breast cancer, but it has not been found in benign breast lesions; this suggests that the gene does not amplify prior to a real malignant state16. In the current series, 21 patients do not show the overexpression of the Her-2 neu gene, upon study through immunohistochemistry (Herceptest) and through fluorescent in situ hybridization (FISH), which is coherent with the low-grade histological types studied.
The proliferation index studied with the Ki 67 antigen permits identifying the proliferating cells within the tumor and the positivity is correlated to the grade of tumor differentiation, vascular invasion, lymph node metastases and it is in inverse proportion to the existence of hormone receptors, as observed in the tumors we have studied in this series.
The advent of new techniques in pathological anatomy like immunohistochemistry (IHC) has increased the sensitivity in detecting micrometastases (10-20%), against H&E stain5. The RT-PCR molecular technique has increased sensitivity from 84 to 93%, and specificity from 95% to 98%, compared to H&E and IHC6.
This study compared the three techniques, reason for which the node was fragmented. Even so, there is high agreement. This has made the molecular technique known with great reliability, which is why currently the study is conducted of 100% of the sentinel node, while with H&E a fragment of the node is studied in the intraoperative. This has impact upon the survival, time free of the disease, therapeutic management, and quality of life of patients with breast cancer.
Conclusions
- This series compared three study techniques of the sentinel node (hematoxylin and eosin, immunohistochemistry, and molecular) and a high correlation was found.
- The molecular technique differentiates between a microor macro-metastasic mass and the lymph node and permits the surgeon to make a definitive decision during the intervention.
- Patients with low-grade, histological-type carcinomas have high survival rates, less aggressive tumor behavior, and lower lymph node commitment during diagnosis and this was the case for the patients in this series.
Conflict of interests. The authors declare having no conflict of interests with the Hospital Clínico San Carlos de Madrid.
References
1. Kuijt GP, Voogd AC, van de Poll-Franse LV, Scheijmans LJ, van Beek MW, Roumen RM. The prognosis significance of axillary lymph node micrometastases in breast patients. Eur J Surg Oncol. 2005; 31: 500-5.
2. Mark K, Hansen N, McMaster K. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Am J Surg. 2004; 188: 49-61.
3. Rivers A, Hansen N. Axillary management after sentinel lymph node biopsy in breast cancer patients. Surg Clin North Am. 2007; 87: 365-7.
4. Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, et al. The sentinel node in breast cancer. A multicenter validation study. N Engl J Med. 1998; 339: 941 56.
5. Gusterson B. The New TNM classification and micrometastases the breast. Breast. 2003; 12: 387-90.
6. Mitas M, Mikhitarian K, Walters C, Baron PL, Elliott BM, Brothers TE, et al. Quantitative real-time RT-PCR Detection of breast cancer micrometastasis using a multigene marker panel. J Cancer. 2001; 93: 162-71.
7. Heidelberg C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, et al. Fluorinated pyrimidines, a new class of tumor inhibitory compounds. Nature. 1975; 179: 663-6.
8. Bland K, Copeland E. The breast: Comprehensive management of benign and malignant diseases. Chapter one. 4a ed. Philadelphia: Elsevier Inc; 2009.
9. Leidenius MH, Vironen JH, Riihelä MS, Krogerus LA, Toivonen TS, von Smitten KA, et al. The prevalence of non-sentinel node metastases in breast cancer patients with sentinel node micrometastases. Eur J Surg Oncol. 2005; 31: 13-28.
10. Smillie T, Hayashi A, Rusnak C, Dunlop W, Donald J, Van der Westhuizen N, et al. Evaluation of fe asibility and accurancyaccuracy of sentinel node biopsy in early breast cancer. Am J Surg. 2001; 181: 427-30.
11. Krontiras H, Bland K. When is sentinel node biopsy for breast cancer contraindicated. Surg Oncol. 2003; 12: 207-10.
12. Leong A. The prognostic dilemma of nodal micrometastases in breast carcinoma. Cancer Chemother. 2000; 27: 315-20.
13. Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, et al. Sentinel node biopsy to avoid axillary dissection in breast with clinically negative lymph nodes. Lancet 1997; 349: 1864-7.
14. Mason Barbara, Holdaway Ian, Mullins Peter, Yee Lye, Kay R. Progesterone and estrogen receptors as prognostic variables in breast cancer. Cancer Res. 1983; 43: 2985.
15. McGuire W, De la Garza M. Similarity of estrogen receptors in human and rat mammary carcinoma. J Clin Endocr Metab. 1973; 36: 548-52.






