Overview of short bowel syndrome and intestinal transplantation
Debora Duro, M.D., M.S.*, Daniel Kamin, M.D.*
* Department of Pediatric Gastroenterology and Nutrition, Children Hospital Boston, Harvard Medical School, Boston, Massachusetts, USA. e-mail: debora.duro@childrens.harvard.edu
Recibido para publicación diciembre 28, 2006 Aceptado para publicación enero 25, 2007
SUMMARY
Short bowel syndrome is at once a surgical, medical, and a disorder, with potential for life-threatening complications as well as eventual independence from artificial nutrition. Navigating through the diagnostic and therapeutic decisions is ideally accomplished by a multidisciplinary team comprised of nutrition, pharmacy, social work, medicine, and surgery. Early identification of patients at risk for long-term PN-dependency is the first step towards avoiding severe complications. Close monitoring of nutritional status, steady and early introduction of enteral nutrition, and aggressive prevention, diagnosis and treatment of infections such as line sepsis, and bacterial overgrowth can significantly improve prognosis. Intestinal transplantation is an emerging treatment that may be considered when intestinal failure is irreversible and children are suffering from serious complications related to TPN administration.
Keywords: Short bowel syndrome; Intestinal transplantation; Children.
Actualización sobre síndrome de intestino corto y transplante intestinal
RESUMEN
El síndrome de intestino corto es una entidad médico-quirúrgico, con potencial riesgo para poner en peligro la vida de los niños, y que en su manejo incluye nutrición artificial. El estudio diagnóstico y terapéutico se logra idealmente con un equipo multidisciplinario compuesto de nutricionista, químico, trabajadora social, médico y cirujano. Uno de los primeros pasos, es la identificación anticipada de pacientes a riesgo de presentar complicaciones severas por el uso prolongado de nutrición parenteral. Su pronóstico se mejora con la estrecha supervisión del estado nutricional, por la introducción temprana de la nutrición enteral y la prevención a tiempo en el diagnóstico y tratamiento de infecciones bien de la línea arterial, o por sobrecrecimiento bacteriano. El transplante intestinal emerge como parte del tratamiento que puede ser considerado cuando la falla intestinal es irreversible y en los niños que presentan complicaciones serias relacionadas con la administración de nutrición parenteral.
Palabras clave: Síndrome de intestino corto; Transplante intestinal; Niños.
The small bowel is completely formed by 20 weeks of gestation. Most of its growth occurs in the 3rd trimester gestation and increases to approximately 250 cm with a diameter of 1.5 cm after 35 weeks of gestation1. An adult small intestine is 600 to 800 cm in length and 4 cm in diameter. The mucosal surface area increases with age; an average infant’s intestine is about 950 cm2 compared with an adult intestine of 7500 cm2. Normal intestinal growth and development are uniquely important for understanding the pathophysiology of pediatric Short Bowel Syndrome (SBS). For instance, the age of the child at the time of intestinal resection may crucially impact the potential for remaining bowel to adapt2. The classic prognostic factors in SBS include the length and site of resection, underlying intestinal disease, status of other digestive organs, and adaptive capability of remaining intestine3.
The nutritional management begins in the early postoperative period. The goals should be towards improving overall quality of life by maintaining normal nutritional status and preventing complications associated with SBS.
This article discuss briefly an overview in SBS presenting the definition, etiology, pathophysiology, complications and the overall management including some considerations for surgical approach and intestinal transplantation.
DEFINITION OF SHORT BOWEL SYNDROME AND ITS ETIOLOGY
There is no specific anatomical definition. By convention, animal studies often use 80% resection or greater to define SBS. In the pediatric population the definition most used and accepted is based on function. SBS is a malabsorptive state occurring as a result of loss of a significant portion of the intestine for acquired or congenital diseases leading to dependence on parenteral nutrition for 1-3 months.
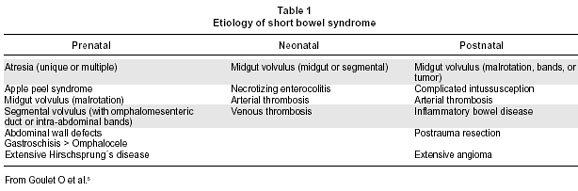
The incidence and prevalence of SBS are estimated to be 3 per million and 4 per million, respectively4. These numbers reflect almost exclusively those individuals requiring parenteral nutrition and are based on numbers in studies done in Europe. Short Bowel Syndrome is the predominant cause of intestinal failure in children and is related to congenital causes such as atresias, gastroschisis or acquired conditions including volvulus, and necrotizing enterocolitis5,6 (Table 1).
PATHOPHYSIOLOGY
The pathophysiology of SBS depends on the extent of the resection and the location of residual small bowel or colon. There is no anatomical distinction that demarcates jejunum from ileum. The proximal 2/5 of small bowel is usually accepted as jejunum and the distal 3/5 as ileum. Most carbohydrates, fats, proteins, vitamins, minerals and trace elements are absorbed within the first 2/3 of the small bowel. Most iron is absorbed in the duodenum and folate in the proximal jejunum. Vitamin B12 and bile salts are only absorbed in the distal ileum. Water and electrolytes are absorbed through the entire small bowel and colon.
The ileum is the most common intestinal segment to be resected. Intestinal transit may be rapid due to a loss of the ileal and colonic7 break making diarrhea a common complication. With loss of terminal ileum, choleretic diarrhea may occur due to bile salt malabsorption. However, even with extensive ileal resections, calorie and fluid absorption may be adequate since these functions occur largely in the jejunum.
Although uncommon, jejunal resections carry the best prognosis. The ileal brake maintains normal intestinal transit so that diarrhea is less common.
The loss of the ileal cecal valve (ICV) may have consequences. The ICV functions as a major barrier to reflux of colonic material from the colon into the small intestine, and assists in regulating the exit of fluid and nutrients from the ileum into the colon. Very common complications can occur with loss of ICV such as bacterial overgrowth and difficulty of weaning from parenteral nutrition8.
COMPLICATIONS AND OVERALL MANAGEMENT
The most important SBS complications relate to the need to administer central venous parenteral nutrition9. Liver disease may develop, and is characterized by steatosis, cholestasis and even cirrhosis. Central venous catheter complications may occur, such as catheter breakage, central venous thrombosis, and catheter-related bacterial or fungal sepsis. Other common complications depend on the length, nature, and surgical anatomy of the remaining small bowel. Malabosrptive diarrhea, fluid and electrolyte abnormalities, micronutrient deficiencies, gastric hypersecretion, anastomotic ulcers and bacterial overgrowth8 all can occur in children with SBS. These children require careful ongoing monitoring and treatment even in a light of normal somatic growth and or limited resected segment of bowel10.
Medical management ought to focus on nutrition, which includes monitoring the provision of calories, micronutrients, fluid, and electrolytes. Usually patients require parenteral nutrition for a period of time. Most can be successfully transitioned to full enteral nutrition11. The gold standard for success is growth once completely off parenteral nutrition, as well as the maintenance of normal vitamin nutriture and liver function12.
Many times children with SBS require medications to help overcome some of the complications associated with SBS. Gastric acid hypersecretion can impair absorption of nutrients and precipitate diarrhea; acid blockade with proton pump inhibitors can be useful in this regard. Multiple mechanisms motivate the use of loperamide, fiber, octreotide, and cholestyramine for the control of voluminous and watery stool or ostomy output. In patients with prolonged exposure to parenteral nutrition, ursodeoxycholic acid may hasten improvement in the cholestasis. Bacterial overgrowth often necessitates the use of rotating courses of enteral antibiotics. The supplementation of vitamins and minerals, especially the fat soluble vitamins A, D, E and K, is fundamental for the preservation of nutritional status in children with SBS.
SURGICAL CONSIDERATIONS
Often surgery is the most appropriate therapy to achieve full enteral nutrition. The most common is the placement of feeding device directly into the gastrointestinal (GI) tract. Typically this is a gastrostomy tube, but gastrojejunal or jejunostomy tubes also play a role for patients with abnormal gastric and/or duodenal motility. The primary purpose of such tubes is the continuous administration of enteral nutrition. Continuous, steady administration of enteral nutrition is more likely to be tolerated than oral bolus feeding in children with SBS13.
Often children with SBS have small intestinal ostomies even while colon may also be present but ‘not in continuity’, i.e. chyme does not pass through a given segment of intestine. As soon as it is surgically and medically appropriate, such segments should be utilized by ‘taking down’ ostomies and allowing intestinal contents to have maximum contact time with small and large intestine. This gives the GI tract the best chance to absorb nutrients, fluid, and electrolytes.
Intestinal lengthening procedures take advantage of the bowel dilation that often occurs in the foreshortened remaining small intestine. LILT, or longitudinal intestinal lengthening and tailoring, was described in 198014, and has now been employed widely. This procedure divides symmetrically dilated segments of small bowel in half longitudinally, preserving blood flow by separating the leaves of mesentery with either limb. The lumen is re-created by forming two narrower channels, which are then re-approximated one to the other in series, effectively doubling the length of the intestinal lumen. Results have been favorable15.
The serial transverse enteroplasty (STEP) procedure was more recently described16, and has now been performed widely and was used recently to treated some specific complications17. It has the advantage of being simpler, requires no enterotomies, preserves natural intestinal vasculature, and can be applied to asymmetrically dilated segments of bowel. The procedure entails applying a surgical stapler at right angles to the bowel successively, alternating sides, so as to create a ‘zig-zag’ longer and narrower channel. A recently created STEP Registry18 reported that enteral tolerance increased by 116% in 38 patients, and nearly half had been weaned off TPN after a median follow-up of 12.6 months.
INTESTINAL TRANSPLANTATION
Intestinal transplantation is indicated when intestinal failure is considered permanent, and the administration of TPN is resulting in life-threatening complications. This has been operationally defined as:
Significant liver injury with abnormal hepatic enzymes.
Multiple central line infections.
Thrombosis of at least two central veins.
Frequent severe episodes of dehydration19.
The most common intestinal transplants can be categorized as follows:
Isolated intestine- transplantation of the small intestine with or without the large intestine;
En bloc liver intestine- the duodenum, pancreas, liver, and small intestine are included ‘in one piece’ so as not to disrupt the biliary tract;
Multivisceral- removal and replacement of the native foregut and midgut. Graft choice usually depends on the size of the recipient, the presence or absence of significant liver disease, and if there is significant pathology extending beyond the small intestine (e.g. pseudoobstruction affecting stomach and small bowel).
Since its entry into the clinical use in the 1980s20 outcomes after intestinal transplantation have dramatically improved. The average one year survival after intestinal transplant is 80%21, and at some centers this value exceeds 90%. Chronic parenteral nutrition is costly and burdensome22, while average 5 year survival may be as low as 60%21. Transplantation still carries significant morbidity and mortality, patients remain on life long immune suppression, and 5 year survival rates (on average 50%) are sub-optimal19. Nevertheless, current indications have been questioned19,21,22, in anticipation of continued improvements in patient and graft survival. Thus, indications may evolve over the coming years to include children with permanent intestinal failure but without necessarily suffering from severe, life-threatening complications.
REFERENCES
1. Weaver LT, Austin S, Cole TJ. Small intestinal length: a factor essential for gut adaptation. Gut 1991; 32: 1321-1323.
2. Quiros-Tejeira RE, Ament ME, Reyen L, Herzog F, Merjanian M, Olivares-Serrano N, et al. Long-term parenteral nutritional support and intestinal adaptation in children with short bowel syndrome: a 25-year experience. J Pediatr 2004; 145: 157-163.
3. Sondheimer JM, Cadnapaphornchai M, Sontag M, Zerbe GO. Predicting the duration of dependence on parenteral nutrition after neonatal intestinal resection. J Pediatr 1998; 132: 80-84.
4. van Gossum A, Bakker H, de Francesco A, Ladefoged K, León-Sanz M, Messing B, et al. Home parenteral nutrition in adults: a multicentre surveyin Europe in 1993. Clin Nutr 1996; 15: 53-59.
5. Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology 2006; 130 (Suppl): 16-28.
6. Ziegler MM. Short bowel syndrome in infancy: etiology and management. Clin Perinatol 1986; 13: 163-173.
7. Nightingale JM, Kamm MA, van der Sijp JR, Morris GP, Walker ER, Mather SJ, et al. Disturbed gastric emptying in the short bowel syndrome. Evidence for a ‘colonic brake’. Gut 1993; 34: 1171-1176.
8. Kaufman SS, Loseke CA, Lupo JV, Young RJ, Murray ND, Pinch LW, et al. Influence of bacterial overgrowth and intestinal inflammation on duration of parenteral nutrition in children with short bowel syndrome. J Pediatr 1997; 131: 356-361.
9. Colomb V, Fabeiro M, Dabbas M, Goulet O, Merckx J, Ricour C. Central venous catheter-related infections in children on long-term home parenteral nutrition: incidence and risk factors. Clin Nutr 2000; 19: 355-359.
10. Duro D, Jaksic T, Duggan C. Multiple micronutrient deficiencies in a child with short bowel syndrome and normal somatic growth. J Pediatr Gastroenterol Nutr. In press 2007.
11. Andorsky DJ, Lund DP, Lillehei CW, Jaksic T, Dicanzio J, Richardson DS, Collier SB, et al. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr 2001; 139: 27-33.
12. Jeejeebhoy KN. Management of short bowel syndrome: avoidance of total parenteral nutrition. Gastroenterology 2006; 130 (Suppl): 60-66.
13. Weizman Z, Schmueli A, Deckelbaum RJ. Continuous nasogastric drip elemental feeding. Alternative for prolonged parenteral nutrition in severe prolonged diarrhea. Am J Dis Child 1983; 137: 253-255.
14. Bianchi A. Intestinal loop lengthening-a technique for increasing small intestinal length. J Pediatr Surg 1980; 15: 145-151.
15. Bianchi A. Longitudinal intestinal lengthening and tailoring: results in 20 children. JR Soc Med 1997; 90: 429-432.
16. Kim HB, Fauza D, Garza J, Oh JT, Nurko S, Jaksic T. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg 2003; 38: 425-429.
17. Modi BP, Langer M, Duggan C, Kim HB, Jaksic T. Serial transverse enteroplasty for management of refractory D-lactic acidosis in short-bowel syndrome. J Pediatr Gastroenterol Nutr 2006; 43: 395-397.
18. Modi B. The First Report of the International STEP Data Registry: Indications, Efficacy and Complications. J Am Coll of Surg. In press 2007.
19. Abu-Elmagd KM. Intestinal transplantation for short bowel syndrome and gastrointestinal failure: current consensus, rewarding outcomes, and practical guidelines. Gastroenterology 2006; 130 (Suppl): 132-137.
20. Pritchard TJ, Kirkman RL. Small bowel transplantation. World J Surg 1985; 9: 860-867.
21. Ruiz P, Kato T, Tzakis A. Current status of transplantation of the small intestine. Transplantation 2007; 83: 1-6.
22. Sudan D. Cost and quality of life after intestinal transplantation. Gastroenterology 2006; 130 (Suppl): 158-162.