Conduction between left superior pulmonary vein and left atria and atria fibrillation under cervical vagal trunk stimulation
Hou Yuemei, MD, PhD*, Zhang Xiaoqin, MD, PhD*, Song Jianguo, MD*, Na Jina, MD*
* Cardiovascular Research Institute, First Teaching Hospital, Xinjiang Medical University, Urmuqi City, China.
e-mail: houyuemei@sina.com
Received for publication July 11, 2007 Accepted for publication June 26, 2008
SUMMARY
Objective: The effect of cervical vagal trunk stimulation on conduction between left superior pulmonary vein and left atria and atria fibrillation have not been systematically studied. We attempted to investigate the electrical conduction between LA and LSPV in dogs with cervical vagal trunk stimulation and evaluate the possible underline substrates for local reentry and initiation and maintenance of AF.
Methods: 12 mongrel dogs (18-25 kg) underwent cervical vagal trunk stimulation at high-frequency (60 ms, 1-4V) to produce sinus arrest lasting >2 seconds,complete atrioventricular AV blocking or the sinus rate decreasing more than 50%. Left lateral thoracotomy was performed and the heart was exposed in a pericardial cradle. The HRA, LAA, PV-LAJ, LSPVm and LSPVd was locally stimulated (100 ms, 2-8V). ERP of HRA, LAA, PV-LAJ and within LSPV, ERP heterogeneity within LSPV and conduction between left superior pulmonary vein and left atria and atrial fibrillation inducing rate with vagal response was analyzed.
Results: 1. During cervical vagal stimulation, the heart rate was reduced significantly from baseline 156±34 bpm to 75±34 bpm (p<0.05, n=12), ERP of HRA, LAA, PV-LAJ, LSPVm and LSPVd sites was shortened locally in all animals, ERP hetero-geneity (COV-ERP ) was increased from baseline 3±3% to 30±13% (on average, p<0.05; n=12) and inducibility of AF was increased from baseline 49.9% to 70.9% with S1S1stimulation (100 ms, 2-8V) and from baseline 3.7% to 43.5% with S1S2 (5 ms decremented stepwise S1S2 300/200 ms, 2-8V, n=12; 2. LA and PV potentials at LA, PV-LAJ, LSPVm and LSPVd was overlapped during sinus rate and separated remarkably by stimulating at distal part of LSPV (n=9); 3. Unidirectional and bi-directional conduction(n=9), bi-directional decremented conduction (n=6), delayed conduction from LSPVp to LSPVd (n=12) and slow conduction from stimulating signal to local tissue potential (n=9) before onset of AF were recorded by pacing from both LSPV and LA.
Conclusion: Our study strongly suggested that fast ectopic beating, abnormal vagal nerve activation as well as remodeling conduction between left superior pulmonary vein and left atria might are the common underline substrates for local reentry and AF initiation and maintenance.
Keywords: Electrical conduction; Atria fibrillation; Left atria; Left superior pulmonary; Cervical vagal trunk.
Conducción entre la vena pulmonar superior izquierda, la aurícula izquierda y la fibrilación auricular bajo el estímulo del tronco cervico-vagal
RESUMEN
Objetivo: El efecto del estímulo del tronco nervioso cervico-vagal sobre la conducción entre la vena pulmonar superior izquierda (LSPV, siglas en inglés), la aurícula izquierda (LA, siglas en inglés) y la fibrilación auricular (AF, siglas en inglés), no se ha estudiado sistemáticamente. Se intentó investigar la conducción eléctrica entre la LA y la LSPV, con el estímulo del tronco nervioso cervico-vagal y evaluar los posibles substratos subyacentes para la reentrada local, la iniciación y el mantenimiento de la AF.
Métodos: En 12 perros sin raza (18-25 kg), se estimuló el tronco cervico-vagal con alta frecuencia (60 ms, 1-4V) para producir detenimiento sinusal con duración >2 s, completo bloqueo atrio-ventricular (AV) o un descenso superior a 50% en el ritmo sinusal. Se hizo una toracotomía lateral izquierda y se expuso el corazón en la malla pericárdica. Los HRA, LAA, PV-LAJ, LSPVm y LSPVd se estimularon localmente (100 ms, 2-8V). El de HRA, LAA, PV-LAJ y dentro de la LSPV, la heterogeneidad dentro de la LSPV y la conducción entre la vena pulmonar superior izquierda, la aurícula izquierda y la fibrilación auricular que induce un ritmo con respuesta vagal, también se analizaron.
Resultados: 1. Durante el estímulo vago-cervical, el ritmo cardíaco se redujo significativamente desde la línea basal 156±34 bpm a 75±34bpm (p<0.05, n=12); los sitios ERP de HRA, LAA, PV-LAJ, LSPVm y LSPVd se acortaron localmente en todos los animales. La heterogeneidad (COV-ERP) aumentó desde la línea basal 3±3% A 30±13% (en promedio, p<0.05, n012) y la capacidad de ser inducida la AF aumentó desde la línea base 49.8% a 70.9% con un estímulo S1S1 (100 ms, 2-8V) y desde la línea basal 3.7% a 43.5% con S1S25 ms reducido paso a paso S1S2 300/200ms, 2-8V, n=12. 2. Los potenciales de la LA y la PV en LA, PV-LAJ, LSPVm y LSPVd, se sobrepusieron durante el ritmo sinusal y se separaron notablemente por el estímulo en la porción distal de LSPV (n=9). 3. La conducción uni-direccional y bi-direccional (=9), la conducción disminuida (n=6), la conducción retardada de LSPVp a LSPVD (n=12) y la conducción lenta desde la señal estímulo hasta el potencial tejido local (n=9), antes del comienzo de la AF se registraron paso a paso tanto desde la LSPV como desde la LA.
Conclusión: Este estudio sugirió con mucho vigor que los latidos ectópicos, la activación anormal del vago, así como la conducción remodelante entre la vena pulmonar superior izquierda y la aurícula izquierda son substratos comunes subyacentes para el reingreso local, la iniciación y el mantenimiento de la fibrilación auricular.
Palabras clave: Conducción eléctrica; Fibrilación auricular; Aurícula izquierda; Vena pulmonar superior izquierda; Tronco nervioso cervico-vagal.
Atria fibrillation is the most common cardiac arrhythmia, getting more prevalent with age1, and with an increased long-term risk of stroke, heart failure and all-cause motality2. The focal source hypothesis, incorporating automaticity and/or local reentry is consistent with a dominant role for the LA in human AF. Previous studies showed that ectopic beats originating from the PV propagated to the LA with characteristically long conduction time, often with conduction delay or block within the PV or at the PV-LA junction3. In canine models vagal stimulation shortens the effective atria refractory period as well as triggers and maintains AF. However, the detailed conduction patterns between LA and PV and inducibility of AF under abnormal parasysmpathetic activation are poorly understood. We hypothesized that cervical vagal trunk stimulation and fast pacing will produce abnormal conduction from LA across the PV-LA junction to PV, which will play an important role in maintains AF originating from the PV.
METHOD
Surgical preparations. In 12 mongrel dogs (18 to 25 kg), anesthesia was performed with sodium pentobarbital (initial bolus 25 mg/kg IV, 5-10 mg/kg as needed for maintenance, every 2 hours). Intubation and mechanical ventilation with oxygen (2 l/min) was given. Two bipolar electrode catheters were inserted through right jugular vein and femoral vein to both right atria and right ventricular for electrophysiological study. After left lateral thoracotomy, the heart was exposed in a pericardial cradle. Multielectrode catheter was placed along the left superior pulmonary vein, a quadripolar catheter in the HIS, a quadripolar catheter in the left atrial appendage (LAA), and a multielectrode catheter was inserted into the coronary sinus. Surface ECG lead II/aVR and intracardiac tracings were recorded by use of a HELLE Cardio-2000 system, filter setting ranged between 0.1 and 250 Hz, whereas bipolar electrocardiograms were filtered at 30-250 Hz.
Cervical vagal trunk stimulation. For cervical vagal trunk stimulation (CVTS), an self-made bipolar silver electrodes with 2-5 mm distance was inserted into the vagosympathetic trunk in a parallel way. Electrical stimuli was delivered by X-VI type stimulator (East Electronic Company, China) at 60 ms (1000 bpm), the voltage chosen was between 1-4V. Effective CVTS stimulation response was defined as: the sinus arrest lasting >2 seconds or complete atrioventricular AV block, or a 50% reduction in heart rate was used as the definition of adequate vagal response. The effective CVTS stimulation response was kept constantly during entire experiment.
Measurement of ERP, conduction and AF inducement. For measurement of ERP, conduction and inducibility of AF, a ten-polar electrode catheter was placed along LSPV, a bi-polar electrode catheter at high right atria (HRA), and a bi-polar electrode catheter at left atria appendage. The conduction sequences across PV-LA junction, within left superior pulmonary vein and the inducibility of AF were assessed before and after vagal stimulation by 60 ms (1000 bpm) continuous electrical pulse pacing at the LA, middle and distal part of LSPV in 10s, 30s and 60s different duration with 30s interval in each time. Effective refractory period (ERP) of LSPV, PV-LAj, HRA and LAA was investigated by 5 ms decremented stepwise S1S2 200 ms pulse of electrical stimulation, with voltage 1-8 V, two times diastolic threshold. AF inducibility was assessed and recorded before and after cervical vagal trunk stimulating. The AF persistent was defined as: AF induced lasting more than 5 min. AF could be stopped automatically or defibrillated by cardioversion or clamped at left atria appendage if it last more than 30 min.
Statistical analysis. Results are expressed as mean ± standard deviation (SD). Statistical comparisons between groups were done with Studentís t test. An index of ERP heterogeneity for each dog was obtained by calculating the coefficient of variation of the 4 regional ERP values. All data were analyzed in software SPSS 11.5. A 2 tailed value of p<0.05 was considered statistically significant.
All animal studies were approved by the Animal Use and Care Committee of Department of Animal Research Institute of Medical Center, Urumuqi City, Xinjiang.
RESULTS
Heart rate. The average heart rate before vagal stimulation was 156±34 bpm and 75±34 bpm during supramaximal CVTS, (p<0.05 n=12). The heart rate decreased gradually with voltage increased from 1 to 4V, while the heart rate will not be reduced any more once the maximal vagal response reached by 4v stimulating. The maximal vagal response was constant during the entire procedure without fatigue phenomenon.
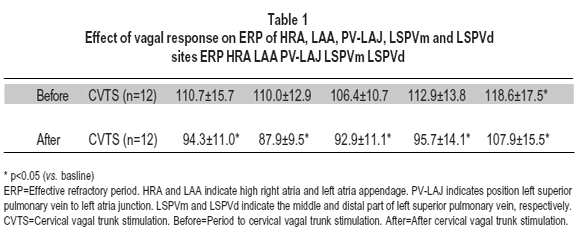
ERP and ERP heterogeneity. The ERP of HRA, LAA, PV-LAJ, LSPVm and LSPVd sites be shortened from baseline on average, 112.8±11.2 ms to 92.6±12.4 ms (p<0.05, n=12) during supramaximal CVTS. The ERP shortening at each site is illustrated in Table 1. Local effective refractory period (ERP) was measured and spatial heterogeneity (dispersion) between each recording sites was evaluated before and after CVTS (dispersion was calculated as the ERP difference between the maximum and minimum value of HRA, LAA, PV-LAJ, LSPVm and LSPVd distant recording sites). The overall conduction vilocity (CV) for each dog was calculated from the average of each of HRA, LAA, PV-LAJ, LSPVm and LSPVd regional CV values. An index of CV heterogeneity in each dog was obtained by calculating the coefficient of variation (COV CV=SD/mean x 100%). The heterogeneity was measured by COV-ERP was 3±3% baseline vs. 30±13%,on average, with CVTS, p<0.05; n=12).
The activation sequence and electrical conduction between LA and LSPV with vagal response:
Inducibility of atria fibrillation. Spontaneous AF could not be induced by CVTS in all 12 dogs. The duration of AF induced varied from animal to animal. On average, the rate of induction of AF was baseline 49.86% with S1S1 100 ms pacing and 3.7% with S1S2 5 ms decremented stepwise pacing at HRA, LAA, LSPV. While the rate of induction of AF could be increased to 70.9% by S1S1 100 ms burst pacing and to 43.5% by S1S2 premature pacing at HRA, LAA, LSPV with vagal response (CVTS by S1S1 60 ms, 1-4V). The distal part of LSPV was the site with lower AF induction rate both at CVTS or without (Figure 4 A, B). Vagal AF usually, but not always, spontaneously converted to sinus rhythm.
DISCUSSION
Currently, ablation strategies for AF consist of pulmonary vein (PV) isolation with or without additional ablation lines in the left atrium and/or targeting the complex electrocardiograms characterized by a short cycle length, fractionation, and/or continuous electric activity. The reported immediate success rate is higher than 90%. The long-term success rate ranges from 60% to 90% in patients with paroxysmal AF, but it is lower in patients with chronic AF4-6. The effectiveness of catheter ablation is related to the precision of the atrial lesions created around the PV which aims to eradicate the triggers, isolate the PV, and modify the substrate for AF maintenance. However, these lesions create large areas of scarring in the atria that might become a substrate for the late onset of atria tachyarrhythmias and depress the left atria contractility7, and they may involve more ablation than necessary in some patients. Further more, ablation along the posterior left atrial wall is associated with the risk of a lethal complication involving an atrioesophageal fistul8. Therefore, new techniques for the ablation of AF are continually evolving.
The heart rate, the ERP, heterogeneity and inducibility of AF with vagal response. Several clinical observations have suggested that an increased parasympathetic tone is involved in the genesis of at least some forms of paroxysmal AF9. Parasympathetic stimulation shortens the AERP and decreases the wavelength of atrial reentrant circuits10. Besides shortening the AERP, vagal stimulation increases the AERP dispersion, which in turn contributes to the stability of AF11,12. Arora et al.13 demonstrate that the PVs, left atrial appendage (LAA), and posterior left atrium (LA) had unique activation and repolarization characteristics in response to autonomic manipulation. They found that vagal stimulation and propranolol plus vagal stimulation caused more ERP shortening in the PV and posterior LA than in the LAA.
In our study, with vagal response simultaneously, we delivered electronic stimulation at dogís cervical vagal trunk and paced at HRA, LAA, PV-LAJ, LSPVm and LSPVd sites, the heart rate was reduced (from 156±34 bpm to75±34 bpm, on average), the ERP in HRA, LAA, PV-LAJ, LSPVm and LSPVd sites was significantly shorted (from 112.8±11.2 ms to 92.6±12.4 ms, on average), and the ERP heterogeneity (COV-ERP) was increased (from 30±1% to 3±3% on average) in almost all 12 dogs. The inducibility of AF (n=12) was increased remarkably (from 49.9% to 70.9% with S1S1 100 ms burst pacing and from 3.7% to 43.5% with S1S2 premature pacing with, respectively). Our results brings the following information:
The conduction between left superior pulmonary vein and left atria with vagal response. Almost 25% paroxysmal AF will develop to chronic AF eventually. Ectopic beats initiating PAF originate from mainly (95%) left pulmonary vein, especially from left superior pulmonary vein (LSPV)14-17. Precisely locating the ablation targets and exactly finding the end point of ablation PV will be certainly useful in reaching higher ablation successful rate, reducing recurrence and complication of AF by pulmonary vein (PV) isolation. Investigating the foci in pulmonary veins, activation conducting characteristics of pulmonary veins will provide us more information of conduction traveling through left atrium to PV-LA and within LSPV.
It has been shown that physiologically the muscular wall of the left atrium may extend up to a few centimeters around the pulmonary veins, more so in the superior than the inferior pulmonary veins. There is one single or dual-input electric conduction back and forth from LSPV, which might produce more than one breakthrough. Previous studies showed that ectopic beats originating from the PV propagated to the LA with characteristically long conduction time, often with conduction delay or block within the PV or at the PV-LA junction18-20. However, the detailed conduction patterns within the PV and across the PV-LA junction with vagal reponse remained unclear.
In our study, with vagal response and by endocardiac mapping, we found that the potentials of LA and PV atLA, PV-LAJ, LSPVm and LSPVd were overlapped with sinus rhythm, were separated by pacing at each sites mentioned above, and were separated remarkably by stimulating at the distal part of LSPV. We also demonstrated that unidirectional (activation conduction sequence was from LA to PV-LAJ, and finally to LSPV by pacing atLA, from LSPV to PV-LAJ, and to LA by pacing at LSPVd) and bi-directional conduction (activation conduction sequence was from LSPVm to both PV-LAJ and LA as well as to LSPVd simultaneously by pacing at LSPVm), bi-directional decremented conduction as well as slowed retrograde conduction (from LSPV to LA) within LSPV and delayed conduction or conduction block from stimulation signal to local tissue potential before AF.
These results remind us activation conduction sequence differs by pacing near LA or within LSPV from that sinus rhythm. The unidirectional, bi-directional and decremented or blocked conducting characteristics within LSPV as well as across PV-LAJ in both with or without vagal response condition remind us that the totally isolation PV form LA in AF ablation will be reached only by blocking activation conduction both in sinus rhythm and also in pacing at PV site.
INDICATION
Our study suggested that the pronounced ERP shortening at PV-LAJ and within LSPVS, significantly increased ERP heterogeneity within LSPV, abnormal activation conduction within LSPV and across PV-LAJ, and different response to cervical vagal trunk vagal stimulation played an important role in local reentry, which might be the underlying mechanism for initiation and maintenance of AF. Our results indicated that vagal denervation, eliminating focal fast firing and blocking activation conduction around PV are the ideal approaches to treat AF and effective way in preventing AF recurrence.
LIMITATION
The present study investigated the electrical conduction characteristics of LA, PV-LAJ and LSPV, ERP of HRA, LAA, PV-LAJ and LSPV, ERP heterogeneity within LSPV and the inducibility of AF by cervical vagal nerve trunk stimulating. We found that the ERP of HRA, LAA, PV-LAJ LSPVm be reduced significantly, ERP heterogeneity within LSPV and the inducibility of AF increased, and the activation conduction sequence at LA, PV-LAJ and within LSPV was different by pacing at LA from that at LSPV. Despite the promising early results, there were some limitations. Firstly, our data came from anesthetized dogs, thus the ERP of LA,PV-LAJ and LSPV, ERP of HRA, LAA, PV-LAJ and LSPV, and the inducibility of AF might be affected on. Secondly, our results could be used only to explain the possible mechanism of AF, which was no structure heart diseases. Finally, we did not pay our attention to sympathetic effect, which might be exist in cervical vagal nerve trunk stimulating.
CONCLUSION
The rapid focal firing within or near LSPV as well as abnormal conduction between LA and PV and within PV under abnormal parasympathetic tone was happy to produce AF. Thus, the higher successful rate of AF ablation, the lower recurrence rate of AF and less complication could be reached only by totally blocked conduction between LA and PV, which could be conformed by comparing the conduction in sinus rhythm with that in pacing at PV site,by eradicating the fast focal firing (triggers) within PV and by modifying autonomic nerve function, which might be the substrate for AF initiating and maintenance. Our research be supported by the grants from Chinese Nature and Science Fund and post-doctor fellowship from The First Teaching Hospital Foundation. Urmuqi City, Xinjiang, China.
REFERENCE
1. Tsang TSM, Miyasaka Y, Barnes ME, Gersh BJ. Epidemiological profile of atrial fibrillation: a contemporary perspective. Prog Cardiovasc Dis. 2005; 48: 1-8.
2. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003; 107: 2920-5.
3. Haissaguerre M, Jais P, Shah DC, Gencel L, Pradeau V, Garrigues S, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998; 339: 659-66.
4. Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001; 104: 2539-44.
5. Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004; 43: 2044-53.
6. Haïssaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005; 16: 1138-47.
7. Gerstenfeld EP, Callans DJ, Dixit S, Russo AM, Nayak H, Lin D, et al. Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation. 2004; 110: 1351-17.
8. Pappone C, Oral H, Santinelli V, Vicedomini G, Lang CC, Manguso F, et al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004; 109: 2724-6.
9. Arora R, Ng J, Ulphani J, MylonasI, Subacius H, ShadeG, et al. Unique autonomic profile of the pulmonary veins and posterior left atrium. J Am Coll Cardiol. 2007; 49: 1340-8.
10. Coumel P. Role of the autonomic nervous system in paroxysmal atrial fibrillation. In: Touboul PC, Waldo AL, editors. Atrial flutter. Armonk: Futura Publishing Co; 1996. p. 248-61.
11. Pappone C, Oreto G, Rosanio S. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104: 2539-44.
12. Smeets JLRM, Allessie MA, Lammers WJEP, Bonke FI, Hollen J. The wavelength of the cardiac impulse and reentrant arrhythmias in isolated rabbit atrium: the role of heart rate, autonomic transmitters, temperature, and potassium. Circ Res. 1986; 58: 96-108.
13. Zipes DP, Mihalick MJ, Robbins GT. Effects of selective vagal and stellate ganglion stimulation on atrial refractoriness. Circ Res. 1974; 8: 647-55.
14. Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol. 1997; 273: H805-16.
15. Takahashi Y, Jais P, Hocini M, Sanders P, Rotter M, Rostock T, et al. Shortening of fibrillatory cycle length in the pulmonary vein during vagal excitation. J Am Coll Cardiol. 2006; 47: 774-80.
16. Gosselink ATM, Crijns HJGM, Van den Berg MP, van den Broek SA, Hillege H, Landsman ML, et al. Functional capacity before and after cardioversion of atrial fibrillation: a controlled study. Br Heart J. 1994; 72: 161-6.
17. Khan R. Identifying and understanding the role of pulmonary vein activity in atrial fibrillation. Cardiovasc Res. 2004; 64: 387-94.
18. Jongbloed MRM, Dirksen MS, Bax JJ, Boersma E, Geleijns K, Lamb HJ, et al. Atrial fibrillation: Multi-detector row CT of pulmonary vein anatomy prior to radiofrequency catheter ablation-initial experience. Radiology. 2005; 234: 702-9.
19. Hertervig E, Kongstad O, Ljungstrom E, Olsson B, Yuan S. Pulmonary vein potentials in patients with and without atrial fibrillation. Europace, April17,2008.
20. Buch E, Shivkumar K. Exploring the potential of pulmonary vein recordings: can they help elucidate mechanisms of paroxysmal atrial fibrillation? Europace, April17,2008.