Inflammatory response in Colombian children with severe protein-energymalnutrition before and after nutritional intervention*
Claudia Velásquez, MSc1, Carolina Navarro, Bacteriol2, César Muñoz, MSc3, Ángel González, PhSc4
* No external funding was sought for this study. Resources were obtained from the 2009-2010 sustainability program of Universidad de Antioquia.
1. Food Research and Human Nutrition Group, Full Professor, Universidad de Antioquia, Medellín, Colombia.
e-mail: claudia.velasquez@siu.udea.edu.co
2. School of Microbiology, Universidad de Antioquia, Medellín, Colombia. e-mail: nabecaro@gmail.com
3. Medical and Experimental Mycology Group, Corporación para Investigaciones Biológicas (CIB), Medellín, Colombia.
e-mail: cmunoz@cib.org.co
4. Professor, School of Microbiology, Universidad de Antioquia. Medical and Experimental Mycology Group, Corporación para Investigaciones Biológicas (CIB). e-mail: agonzalezm@cib.org.co
Received for publication October 27, 2009 Accepted for publication January 23, 2010
SUMMARY
Aim: To evaluate the changes in C-reactive protein and pro-inflammatory cytokines in severely malnourished children, before nutritional intervention and at the moment of restoring appetite.
Methodology: To assess changes in inflammatory mediators, 20 severely malnourished children under 5 years of age, 10 with kwashiorkor and 10 with marasmus were studied. Hemoglobin, total serum proteins, albumin, ferritin, transferrin, ceruloplasmin, C-reactive protein and pro-inflammatory cytokines (IL-8, IL-1β, IL-6,IL-10,TNF-α, and IL-12p70) were determined.
Results: Upon hospital admission, the mean values of C-reactive protein in kwashiorkor and marasmus patients (16.3±19.0 mg/l and 23.1±27.9 mg/l, respectively) indicated an inflammatory response process with no difference between both groups (p=1.0). Total protein, albumin, transferrin and ceruloplasmin in children with kwashiorkor were significantly lower than in marasmic children (p=0.003, p=0.007, p=0.035, p=0.007, respectively). All cytokines, except IL-12p70, showed significantly higher concentrations in kwashiorkor than in marasmic children. After the stabilization phase, concentrations of C-reactive protein decreased significantly in both groups and albumin increased to normal values, but cytokines remained high.
Conclusion: These results show that malnourished children are able to synthesize C-reactive protein in response to an infectious process. Additionally, higher levels of pro-inflammatory cytokines and depletion of albumin in children with kwashiorkor suggest that these inflammatory mediators could be critical biomarkers during clinical phases of kwashiorkor.
Keywords: Malnutrition; Marasmus; Kwashiorkor; Cytokines; C-reactive protein; Inflammatory response.
Respuesta inflamatoria en niños colombianos con desnutrición grave antes y después del tratamiento nutricional
RESUMEN
Objetivo: Evaluar los cambios en la concentración de proteína C reactiva y citocinas pro-inflamatorias en niños con desnutrición aguda grave antes del tratamiento nutricional y al recuperar el apetito.
Metodología: Se evaluó en 20 niños menores de 5 años con desnutrición aguda grave, 10 con marasmo y 10 con kwashiorkor, el cambio en la respuesta inflamatoria mediante la concentración de ferritina, transferrina, proteínas totales, albúmina, ceruloplasmina, proteína C reactiva y citocinas pro-inflamatorias (IL-8, IL-1β, IL-6,IL-10,TNF-α y IL-12p70).
Resultados: Al momento de la admisión, la concentración promedio de proteína C reactiva en niños con kwashiorkor y marasmo (16.3±19.0 mg/l y 23.1±27.9 mg/l, respectivamente) indicaron un proceso inflamatorio activo en ambos grupos (p=1.0). Las proteínas totales, la albúmina, la transferrina y la ceruloplasmina fueron significativamente menores en niños con kwashiorkor que en niños con marasmo (p=0.003, p=0.007, p=0.035, p=0.007, respectivamente). Todas las citocinas, excepto la IL-12p70, mostraron una concentración significativamente mayor en niños con kwashiorkor que en marasmáticos. Después de la fase de estabilización la concentración de proteína C reactiva disminuyó de manera significativa en ambos grupos y la albúmina aumentó a concentraciones normales, pero las citocinas permanecieron altas.
Conclusión: Estos resultados muestran que los niños desnutridos graves son capaces de sintetizar proteínas de fase aguda como la proteína C reactiva en respuesta a un proceso infeccioso. Adicionalmente, las mayores concentraciones de citocinas pro-inflamatorias y la mayor depleción de albúmina ocurrida en niños con kwashiorkor sugieren que esos mediadores inflamatorios pueden ser biomarcadores críticos durante las fases clínicas del kwashiorkor.
Palabras clave: Desnutrición; Marasmo; Kwashiorkor; Citocinas; Proteína C reactiva; Respuesta inflamatoria.
Between 10 and 20% of the children worldwide suffer protein-energy malnutrition (PEM). Despite great advances in the prevention and treatment of malnutrition, Colombia reported in 2005, a prevalence of 1.2% of acute malnutrition-quite similar to that reported in 2000 (0.8%), indicating that this problem persists in some marginalized areas of our country1.
During malnutrition, there is deterioration of the immune response with serious compromise of lymphoid tissues and cellular immunity2,3. Additionally, a decrease is observed in the proportion of TCD4/TCD8 lymphocytes with a ratio under 0.8, comparable to immunodeficiency stages4.
Along with the deterioration of the immune response, PEM also modifies the acute-phase inflammatory response, in part, by alterations in the production and activity of inflammatory mediators, including cytokines and acute-phase proteins. The findings published on in vitro cytokine production by peripheral blood mononuclear cells (PBMC) from children with PEM are limited and generally show a decline in the ability of these PBMC to produce cytokines such as interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α), which mediate or modulate the acute-phase response5,6. Other studies have shown that severely malnourished children have decreased capacity to produce Th1 cytokines such as IL-2 and IFN-γ; on the other hand, they present augmented ability to produce Th2 cytokines including IL-4 and IL-5, indicating that PEM could diminish the Th1/Th2 ratio7.
In contrast to results found in cellular studies, children with PEM and infections are able to maintain similar blood concentrations (plasma levels) of IL-1, IL-6 and TNF-α compared to controls. The study reported that the concentration of pro-inflammatory cytokines was higher in plasma of malnourished children than in healthy individuals, and higher in children with kwashiorkor than in marasmic children. Nonetheless, it is interesting that children with severe acute malnutrition and with a concurrent infection are able to maintain the serum pro-inflammatory cytokines at adequate levels8.
Researchers have shown that even under the most severe forms of malnutrition, children are able to increase the blood concentration of acute-phase proteins, such as C-reactive protein (CRP) at expense of production of circulating proteins, including albumin and transferrin9. CRP protein is synthesized in the liver and modulated by pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α. Its increase reflects the spread of infectious or inflammatory stage and its decrease represents clinical improvement, as well as the effectiveness of a therapeutic intervention. It is known that increase synthesis of CRP is lower in malnourished than in eutrophic children and even lower in children with kwashiorkor than those with marasmus10.
The «Unidad Vida Infantil» at the Francisco Valderrama Hospital, under advice from Universidad de Antioquia, addresses issues of severely malnourished children (with marasmus or kwashiorkor) in the area of Urabá, Colombia. The deterioration of the immune system and high incidences of coexisting infections are widely documented in these children11. Nutritional interventions were performed by following World Health Organization (WHO) guidelines12. These guidelines suggest the onset of nutritional repletion and supplementation with iron approximately 5 days after admission, i.e., when appetite is restored. This suggestion has been controversial because some research has shown that at this time, malnourished children present some infection processes and have detectable concentrations of iron «free» in the serum that induces oxidative stress and edema, which could complicate their recovery13.
In the present work, we determined the concentration of total protein, albumin, hemoglobin, ferritin, transferrin, ceruloplasmin, CRP and the pro-inflammatory cytokines IL-1β, IL-6, IL-8, IL-10, TNF-α, and IL-12 in children with severely acute malnutrition. This study was initiated upon hospital admission (phase 1, before implementing nutritional interventions), and we assessed the changes in the concentration of inflammatory mediators after appetite was restored and before supplementation with iron (phase 2).
MATERIALS AND METHODS
Subjects. Children under five years of age with severely acute malnutrition with marasmus or kwashiorkor were enrolled in this study. According to the Colombian Ministry of Health, research was classified as minimal risk. The procedures applied to children in the study were considered ideal for their recovery as suggested by WHO. The children’s parents granted consent to participate in the research on a voluntary basis. The project received approval by the Central Committee of Bioethics at Universidad de Antioquia.
In order to determine the individuals’ number for this study, the PRIMER program (PRIMER OF BIOSTATISTICS: THE PROGRAM By Stanton A. Glantz. Copyrigth 1992 by McGraw-Hill. Inc. Version 3.02) was used including the following criteria: a power of 90% and an alpha error of 0.05%. Additionally, we defined IL-6 as a main parameter to compare the groups, we used the study by Dulguer et al.8 as a model, where IL-6 serum levels were determined among children with severely acute malnutrition and eutrophic individuals (the differences were 11.9±9.3 pg/ml). The application of this formula determined a minimum children’s number of 10 to be included per group.
Inclusion and exclusion criteria. Children with severely acute malnutrition with or without apparent infection and with or without anemia were involved in this study. Malnutrition was classified according to the WHO protocol: marasmus, when the child presented a ratio of weight/height (W/H) below -3DS without edema; and kwashiorkor, when they presented edema at least malleolus bilateral independent of the W/H. We excluded children with edema secondary to kidney, heart, liver, and endocrine diseases, as well as children who needed transfusion for anemia and children with severe dehydration.
Before starting the nutritional therapy (at hospital admission), inflammatory mediators were measured in the sera of 2 groups of children with severely acute malnutrition: kwashiorkor and marasmus. This value was considered the baseline (phase 1). Later, when children with malnutrition regained appetite, were considered stable and before supplementation with iron (phase 2), the concentration of variables was again measured, and changes between the two phases were assessed.
In phase 1, we made determinations, including total and differential blood cell counts, urinalysis and parasitological examination of smear blood to investigate the presence of malaria, and chest X-rays were done, among others. Total blood (5 ml) was collected to determine total protein, albumin, transferrin, ferritin, hemoglobin, ceruloplasmin, cytokines and CRP.
METHODS
Diagnosis of associated diseases. A trained physician identified clinical signs of infection such as fever, somnolence, hypoglycemia, hypothermia and specific signs of each disease. A measurement of CRP >8 mg/l was considered an infection marker. Anemia diagnosis was made with concentration of hemoglobin below 11 g/dl.
Anthropometrical measurements. The weight of the children was taken daily by using a mechanical scale (Health Meter ®), with 10-g sensitivity; children were weighed without clothes. Because of the age and health status, the children’s height was measured in the supine position. All children were weighed and measured with the same anthropometric parameters and by trained nurses. Each anthropometric measure was performed three times, and when the difference between data exceeded 10 g in the case of weight, and 2 mm in the length, mean values were reported.
Nutritional therapy. Immediately after each child was evaluated and after collecting blood samples, the nutritional treatment was offered initially every two hours. According to the protocol, we started with the F75 formula containing 75 kcal/100 ml and 0.9 g of protein per 100 ml. When a child showed restored appetite, approximately 5 days after hospital admission and before supplementation with iron a new blood sample was collected to evaluate the variables a second time (phase 2).
Medical treatment. The protocol recommends that all children with severely acute malnutrition must be treated with antibiotics, considering every severely malnourished child as infected although not often presenting signs of infection. Thus, children in this study were treated with ampicillin and/or amikacin. In some cases, and depending on the disease diagnosed, as well as its severity and evolution, the physician used the antibiotic for each specific case. Parasites were treated in children over two years of age via 100 mg of mebendazol; using pyrantel pamoate in children under two years of age.
Clinical evolution. On a daily basis, a physician evaluated the infection course, appetite, and weight of children. Appetite restoration was considered when malnourished children were able to consume more than 130 ml/kg/day of the F75 formula for two days. When it was found that the child regained appetite, before supplementation with iron, a blood sample was ordered to reassess transferrin, ferritin, ceruloplasmin, CRP, and cytokines; this was considered phase 2 of the investigation.
Biochemical assessment. Hemoglobin was determined by the cyanmethemoglobin method, ferritin by micro-particle enzyme immunosorbent assay (MEIA); C-reactive protein by turbidimetry commercial CRP assay (Biosystems); transferrin by turbidimetry assay; ceruloplasmin quantification was done by using nephelometry Array. The serum was stored at -70°C until use.
Pro-inflammatory cytokines determination. Measurement of pro-inflammatory cytokines IL-8, IL-1β, IL-6, IL-10, TNF-α, IL-12p70 was done in serum by using a commercial Human Inflammation Kit (BD™ Cytometric Bead Array) and using flow cytometry, adhering to manufacturer’s recommendations and as described elsewhere14.
Statistical analysis.The assessment was normalized by using the Kolmogorov-Smirnov test. The longitudinal analysis was performed by using ANOVA of repeated measures. The frequency analysis was done with the Freeman Halton test. The correlation between variables was performed with the Pearson Correlation or Spearman Rho tests. Statistical probability of p<0.05 was considered significant. The statistical analysis was performed by using the SPSS® statistical program (Statistical Package for the Social Sciences, Chicago, IL, USA) V 15.0.
RESULTS
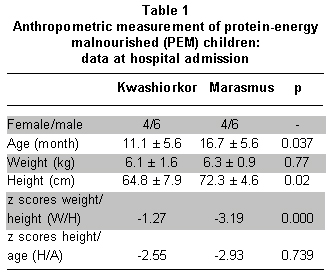
In children with marasmus and kwashiorkor, no significant differences were found among gender, ethnicity, or weight; however, significant differences were observed in age and height, revealing that children with kwashiorkor were smaller (Table 1). Upon admission, none of the children was suffering from malaria, 5 of 10 children suffered kwashiorkor, and 6 of 10 with marasmus showed signs of infection, mainly fever and somnolence without significant differences between them (p=0.5). Diarrhea was observed in 6 of 10 children with kwashiorkor and 5 of 10 children with marasmus. After hospital admission, all children, regardless of the type of malnutrition, received the same medical care and nutritional treatment: feeding F75 formula every two hours, antibiotics, electrolytes, vitamins, minerals, except iron, and trace elements. There was no difference between the two groups of malnourished children in the time needed to stabilize them and to restore their appetite. Furthermore, there was no difference in the ability to ingest food (F75) or consumption of minerals and oligoelements. Both groups ingested equivalent amounts of folic acid supplements (p=1.0) and vitamin A (p=0.144).
On phase 1, the concentrations of total serum proteins, albumin, transferrin, and ceruloplasmin were significantly lower in children with kwashiorkor than in those with marasmus (p=0.003, p=0.007, p=0.035, p=0.007, respectively). In addition, children with kwashiorkor had plasma concentrations of these proteins below the cut-off point. Children with marasmus presented values of albumin and transferrin below the cut-off points. CRP and ferritin showed no statistically significant differences between the two groups (p=1.000, p=0.912, respectively), and their concentrations were above the normal-range limits. Hemoglobin indicated anemia in both groups without differences between them (p=0.143) (Table 2, pb).
After stabilizing the patients, phase 2, giving the protocol for about 5 days, when children with malnutrition regained again appetite and before supplementation with iron, we observed that any, of these proteins differed significantly between the two groups (Table 2, pc). Therefore, we observed a significant decrease in CPR concentration in both groups when comparing the concentrations upon admission and after the nutritional treatment was administered. Simultaneously, it was observed that albumin, ceruloplasmin, and transferrin increased in children with kwashiorkor and in children with marasmus (Table 2, pa y p*a).
During phase 1, all cytokines studied, except for IL-12p70, were significantly higher in children with kwashiorkor than in children with marasmus; nonetheless, during phase 2 significant differences were observed in TNF-α and IL-10 levels were higher in children with kwashiorkor (Table 3). In addition, it was observed that none of the cytokines evaluated changed significantly their concentrations between phase 1 and phase 2 in either group (Table 3, pa y p*a).
DISCUSSION
PEM associated mainly with the presence of infections remains a determining factor in infant mortality in developing countries. In the present study, 60% of children with severely acute malnutrition had diarrhea or other signs of infections, similar to the statistics of African and Asian countries and other studies done in Colombia11. However, severely malnourished children can develop infections without showing signs because of the immune-suppression process. In malnourished children, it has been documented that they had altered defense mechanisms during an early infectious process, which was necessary to induce an acute-phase response with increased synthesis of inflammatory mediators such as cytokines and acute-phase proteins including CRP15.
At the time of admission, phase 1, the concentration of the acute-phase proteins indicated that the malnourished children were able to respond to inflammatory insult with increased concentrations of CRP and ferritin, this difference was not significant between children with kwashiorkor and marasmus. Consistent with these findings, Manary et al.16, observed increases in CRP with no differences between the two types of severe malnutrition; nonetheless, Amesty-Valbuena et al.10 found high CRP levels in children with marasmus. These studies agree that severely malnourished infected children are capable of increasing concentrations CRP in response to infectious diseases.
In contrast, upon admission, some parameters like total proteins, albumin, transferrin, and ceruloplasmin were significantly lower in children with kwashiorkor than in marasmic, and their concentrations in both groups were below the reference value, indicating a depletion process. Our results are consistent with other studies in which children with kwashiorkor also had a higher depletion of total proteins and albumin, which are clinically associated with edema9. The increase of acute-phase proteins like CRP and ferritin with simultaneous decrease of proteins like albumin and transferrin are related to adaptation processes developed by malnourished children to respond against inflammatory challenge, allowing a controlled spread of an infectious process.
Researchers have found that severely malnourished children presented concentrations of IL-1, IL-6, and TNF-α, key mediators of the acute-phase response, similar to healthy eutrophic controls, indicating that the malnourished children were able to produce cytokines that induce an acute-phase response, but the magnitude of this response is poor17. Abo-Shousa et al.5 found that in severely malnourished children serum levels of IL-8 and IL-6 increased compared with healthy controls, indicating that the former were able to respond to an inflammatory stimulus as a product of several infections experienced by children with PEM.
A compensated malnourished child without infection presents a low rate of synthesis and protein degradation (a lower protein replacement), which allows saving protein and energy to supply food deficiency and promote survival. Even so, the presence of infection alters such processes by accelerating protein replacement to provide amino acids necessary to achieving an appropriate acute-phase response. This response requires a high production of cytokines and CRP, which occurs at the expense of albumin and transferrin concentrations. Accordingly, the infection process could be a trigger for the edema formation in kwashiorkor. Thus, the results of our study support this hypothesis in children with kwashiorkor, correlating the high concentration of CRP, ferritin and pro-inflammatory cytokines with low availability of proteins such as albumin and the development of edema. However, Jahoor F et al.18 found that when there is chronic food deprivation, children with marasmus can maintain body protein breakdown at the same rate as when they are well nourished, but children with edema cannot. The slower protein breakdown rate of children with edema reduces the supply of most amino acids, resulting in decreased availability for the synthesis of plasma proteins involved in nutrient transport and the acute-phase response to infection.
In phase 2, after children were stabilized and their appetite restored, a significant decrease in CRP concentration in both groups was observed with a simultaneous increase in the concentration of albumin, transferrin, and ceruloplasmin; in addition, the levels of these proteins changed from an initial deficiency state to concentrations within the normal reference values. Similar results were described by Reid et al.9, which observed decrease in CRP concentrations of children with marasmus and edema during two different moments.
The increase in concentrations of albumin, transferrin, and ceruloplasmin between phase 1 and phase 2 was higher in children with kwashiorkor than in children with marasmus, showing faster recovery in edema. Similar results were described by Jahoor et al.19, who assessed the endogenous leucine flux, an index of whole-body protein breakdown rate. They found that breakdown rate was slower (p<0.01) in the edematous group than in the non-edematous group in the malnourished state; but in the recovered state, it was faster in the children who were previously edematous (p<0.05). When compared with the malnourished state value, breakdown rate at recovery doubled in the group that were previously edematous, but it did not change in the other group.
Although no significant differences were observed, the cytokine levels were higher upon admission than when children recovered appetite; nonetheless, these levels remained high, indicating that the inflammatory response was not totally resolved. It is important to note that the pro-inflammatory cytokines and the most important mediators of the acute-phase response, such as IL-1, IL-6, IL-8, and TNF-α virtually did not change between the first and second phases in either group. The IL-12 had the highest values of all IL in both phases, with no differences between groups, it is critical to generate Th1 responses that provide protection against parasitic infections common in the area. The IL-12 promotes differentiation from LT CD4+ to Th1 cytokine pattern, inducing synthesis of IL-2 and TNF-α20; their highest concentrations in children with kwashiorkor may explain the increased levels of pro-inflammatory cytokines in phase 1. In phase 2, the IL-12 level remained high in both groups, which could explain why the other IL did not decrease their concentrations between the first and second moment. The IL-10, known as Th3 cytokines with regulatory properties by inducing Th2 response and suppressing production of Th17, reached the highest concentrations in both phases in the group with kwashiorkor, possibly in an attempt to decrease the secretion of pro-inflammatory cytokines (IL-1, IL-6, IL-8, TNF-α); however, its concentration was always less than that achieved by IL-12, so that it could prevail in the balanced TH1 stimulation.
Taken altogether, the above results reveal that malnourished children in both groups responded to inflammatory insult by increasing CRP concentrations without significant differences between them. Children with kwashiorkor presented the greatest depletion of total proteins and albumin, which is clinically associated with edema; in addition, they had significantly higher concentrations of IL-1β, IL-6, IL-8, IL-10, and TNF-α in comparison with those with marasmus. After they recovered their appetite, considered as the stabilization phase, children showed significant reduction in CRP and increase in albumin; this indicates that the proteins begin to be recovered and that the inflammatory response possibly starts to be resolved, although cytokines did not diminish significantly even after starting treatments with antibiotics and after stabilizing the patients and their edema. More studies are needed to investigate the precise time frame where the pro-inflammatory cytokines could serve as markers of the infectious process in children with PEM. Such information also would assist in implementing timely nutritional interventions, including iron supplementation therapies.
Conflict of interest. None of the authors has conflicts of interest related to this study.
REFERENCES
1. Instituto Colombiano de Bienestar Familiar, PROFAMILIA, Instituto Nacional de Salud, Universidad de Antioquia, Organización Panamericana de la Salud. Valoración del estado nutricional por indicadores antropométricos. In: Encuesta nacional de la situación nutricional en Colombia, 2005. Bogotá, DC: ICBF; 2006. p. 69-120.
2. Cunningham-Rundles S, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005; 115: 1119-28.
3. Savino W, Dardenne M, Velloso L, Silva-Barbosa S. The thymus is a common target in malnutrition and infection. Br J Nutr. 2007; 98 (Suppl 1): 11-6.
4. Ziegler TR, Evans ME, Fernández-Estivariz C, Jones DP. Trophy and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr. 2003; 23: 229-61.
5. Abo-Shousha SA, Hussein MZ, Rashwan IA, Salama M. Production of pro-inflammatory cytokines: granulocyte-macrophage colony stimulating factor, interleukin-8 and interleukin-6 by peripheral blood mononuclear cells of protein energy malnourished children. Egypt J Immunol. 2005; 12: 125-31.
6. Giovvambattista A, Spinedi E, Sanjurio A, Chisari A, Rodrigo M, Pérez N. Circulating and mitogen induced in tumor necrosis factor (TNF) in malnourished children. Medicina (B Aires). 2000; 60: 339-42.
7. Rodríguez L, González C, Flórez L, Jiménez-Zamudio L, Graniel J, Ortiz R. Assessment by flow cytometry of cytokine production in malnourished children. Clin Diagn Lab Immunol. 2005; 12: 502-7.
8. Dulger H, Arik M, Sekeroglu MR, Tarakcioglu M, Novan T, Cesur Y et al. Pro-inflammatory cytokines in Turkish children with protein-energy malnutrition. Mediators Inflamm. 2002; 11: 363-5.
9. Reid M, Badaloo A, Forrester T, Morlese JF, Heird WC, Jahoor F. The acute-phase protein response to infection in oedematous and nonoedematous protein-energy malnutrition. Am J Clin Nutr. 2002; 76: 1409-15.
10. Amesty-Valbuena A, Pereira N, Castillo J, García D, Nuñez J, Cayama N et al. Mediadores de inflamación (proteina C reactiva) en el niño con desnutrición proteico-energética y en el niño eutrófico. Invest Clin. 2004; 45: 53-62.
11. Bernal C, Velásquez C, Alcaraz G, Botero J. Treatment of severe malnutrition in children: experience in implementing the World Health Organization Guidelines in Turbo, Colombia. J Pediatr Gastroenter Nutr. 2008; 46: 322-8.
12. World Health Organization. Management of the child with serious infection or severe malnutrition. Guidelines for care at the first-referral level in developing countries (WHO/FCH/CAH/00.1). Geneva: WHO; 2000. URL: http://www.who.int/child_adolescent_health/documents/fch_cah_00_1/en/
13. Parra B, Velásquez C, Agudelo G, Cardona O, Betancur M, Morales G et al. Cambios en la concentración sérica de hierro «libre» en niños con desnutrición aguda grave bajo tratamiento de recuperación nutricional. Turbo, Colombia. Perspectivas en Nutrición Humana. 2005; 14: 29-45.
14. Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000; 243: 243-55.
15. Scherbaum V, Furst P. New concepts on nutritional management of severe malnutrition: The role of protein. Curr Opin Clin Nutr Metab Care. 2000; 3: 31-8.
16. Manary MJ, Broadhead RL, Yaresheski KE. Whole-body protein kinetics in marasmus and kwashiorkor during acute infection. Am J Clin Nutr. 1998; 67: 1205-9.
17. Azevedo ZMA, Victal L, Fonseca K, Camara F, Haeffner-Cavaillon N, Cavaillon JM et al. Increased production of tumor necrosis factor-a in whole blood cultures from children whit primary malnutrition. Braz J Med Biol Res. 2005; 38: 171-83.
18. Jahoor F, Badaloo A, Reid M, Forrester T. Protein metabolism in severe childhood malnutrition. Ann Trop Paediatr. 2008; 28: 87-101.
19. Jahoor F, Badaloo A, Reid M, Forrester T. Protein kinetic differences between children with edematous and nonedematous severe childhood undernutrition in the fed and postabsorptive states. Am J Clin Nutr. 2005; 82: 792-800.
20. Jiang H-R, Muckersie E, Robertson M, Xu H, Liversidge J, Forrester JV. Secretion of interleukin-10 or interleukin-12 by LPS-activated dendritic cells is critically dependent on time of stimulus relative to initiation of purified DC culture. J Leukoc Biol. 2002; 72: 978-85.