Determination of the prevalence of hemoglobin S, C, D, and G in neonates from Buenaventura, Colombia*
Matilde de Bernal, MD1, Andrés Collazos, MD2, Rubén Darío Bonilla, Biol3, Edna Patricia Tascón, Biol4
* Resources from the Endocrinology Laboratory at Universidad del Valle and co-financing through resources from an internal call for research projects at Universidad del Valle.
1. Full Professor, Department of Internal Medicine, Endocrinology Laboratory, Universidad del Valle, Cali, Colombia.
e-mail: endocrino@univalle.edu.co
2. Assistant Professor, Universidad del Valle, Department of Internal Medicine, Universidad del Valle, Cali, Colombia.
e-mail: andrescollazos_md@yahoo.com
3. Biologist, Department of Internal Medicine, Endocrinology Laboratory, Universidad del Valle, Cali, Colombia.
e-mail: dbonilla@univalle.edu.co
4. Biologist, Universidad del Valle, Cali, Colombia. e-mail: eptp04@gmail.com
Received for publication December 7, 2009 Accepted for publication January 12, 2010
SUMMARY
Introduction: Inherited hemoglobinopathies are common among African Blacks. In Buenaventura, a city on Colombiaís Pacific coast, where 92% of the population is Afro-Colombian, there are few published attempts to identify these disorders. Affected individuals require more health care due to higher morbidity and mortality. Early identification of these newborns followed by comprehensive care is important to reduce co-morbidities.
Objective: To study newborns and establish the numbers at risk with a bloodspot screening method. This information will demonstrate to Public Health Authorities the need to provide care for this population.
Methods: A cross-sectional descriptive study of a sample of 399 newborns (95% CI) where there is an expected prevalence of 10% of abnormal hemoglobins. Mothers in at least the 36th week of gestation, living in the urban area of Buenaventura, were used. Umbilical cord blood was drawn and specimens fixed on filter paper and stored at 4°C. Isoelectric focusing electrophoresis assays were used to separate the hemoglobins. The results were reported according to the identified hemoglobin as F, A, S, C, D, and G.
Results: We processed 399 samples, 353 (88.5%) were normal (hemoglobin FA), 23 (5.8%) were heterozygous for hemoglobin C (FAC), 19 (4.8%) were heterozygous for hemoglobin S (FAS), 2 (0.5%) were heterozygous for hemoglobin G (FAG), 1 was heterozygous for hemoglobin D (FAD) and 1 was heterozygous combined S and C (FSC).
Conclusion: Hemoglobins S, C, D, and G are common among infants born in Buenaventura. Hemoglobin C occurred more frequently than in other reported studies. This study suggests that both detection and a follow-up program are required in areas with a high density of Afro-Colombian population.
Keywords: Prevalence; Newborn; Hemoglobinopathies; Hemoglobin S; Hemoglobin C; Anemia; Sickle cell; Electrophoresis, iso electric point; Colombia.
Determinación de la prevalencia de la hemoglobina S, C, D y G en recién nacidos de Buenaventura, Colombia
RESUMEN
Introducción: Las hemoglobinopatías son entidades muy frecuentes en el África negra. En Buenaventura, ciudad de la costa pacífica colombiana con una población 92% afrocolombiana, hay pocas publicaciones identificando esta patología. Las poblaciones afectadas requieren cuidados especiales por su alta mortalidad y morbilidad. La identificación temprana desde el periodo neonatal y los programas de cuidado integral se imponen en poblaciones de alta prevalencia.
Objetivo: Determinar la prevalencia en Buenaventura de hemoglobinas S, C, D y G con un modelo de tamizaje neonatal. La información colectada servirá para sustentar recomendaciones a las autoridades de salud pública.
Metodología: Se realizó un estudio descriptivo de corte transversal en 399 neonatos para una prevalencia esperada del 10% (IC 95%), con madres residentes del área urbana de Buenaventura y 36 semanas o más de gestación. Se tomó muestra de sangre de cordón umbilical fijada en papel filtro y almacenada a 4°C. La técnica de electroforesis de punto isoeléctrico sirvió para la separación de las hemoglobinas. Los resultados se informaron de acuerdo a las hemoglobinas identificadas F, A, S, C, D y G.
Resultados: Se procesaron 399 muestras de cordón. El 88.5% (353) de las muestras fueron normales (hemoglobina FA), 23 (5.8%) fueron heterocigotos para hemoglobina C (FAC), 19 (4.8%) fueron heterocigotos para hemoglobina S (FAS), 2 (0.5%) fueron heterocigotos para hemoglobina G (FAG), 1 fue heterocigoto para hemoglobina D (FAD) y 1 fue heterocigoto combinado S y C (FSC).
Conclusión: Hemoglobinas S, C, D y G son frecuentes entre los neonatos de Buenaventura. La hemoglobina C fue más frecuente que en estudios previos, probablemente por sesgos en la selección de la muestra de dichos estudios. Se sugiere a las autoridades de salud pública la ampliación del programa de tamizaje de enfermedades congénitas como las hemoglobinopatías.
Palabras clave: Prevalencia; Recién nacido; Hemoglobina S; Hemoglobina C; Anemia de células falciformes; Electroforesis de punto isoeléctrico; Colombia; Hemoglobinopatías.
Neonatal screening for abnormal hemoglobin (Hb) permits establishing the presence of hemoglobinopathies in the population. Sickle-cell disease is one of these, with the disorder being most frequent in countries with obligatory neonatal screening1,2. It is produced by the specific mutation of the beta-globin gene3, resulting from the change of glutamic acid for valine in the 6th position, of autosomal recessive inheritance. The most common genotypes are: homozygous hemoglobin S (HbS), combined heterozygous SC (HbSC), and Sβ+ thalassemia and Sβ0 thalassemia; and, less frequently, it is due to the combination of hemoglobin S with other variants (for example, hemoglobin D, G). Sickle-cell disease increases morbidity and mortality uring the first years of life4, being greater in homozygous SS individuals in comparison to heterozygous SC. Hemoglobin C is associated to less symptoms and offers protection against severe malaria5.
The high prevalence of sickle-cell anemia in a population with African ancestry was shown in the analysis of records from Texas from 1992 to 1998, from 2í292,698 live births and 94% specimens collected, revealing total prevalence per ethnic groups per every 10,000 live births of 29.91 Afro-Americans, 0.11 White, 0.29 Hispanic, and 2.47 others6. The studies conducted in the city of Buenaventura and in other towns in the Colombian Pacific region are: from a reference center for malaria infection in individuals older than one year of age that showed a prevalence of 14.9% of abnormal hemoglobins (AS of 7%, AC of 6.2%, and AD of 1.7%)7; from the Hematology service at Universidad del Valle in individuals referred because of having samples suspicious of sickle-cell anemia8, and from Salahonda (close to Tumaco in Nariño, Colombia) with sickle-cell trait in 10% of the population and clinical relevant hemoglobinopathies by 1%9.
A preliminary report published in 2004 of neonatal screening begun in Cali (Valle del Cauca, Colombia, with an Afro-Colombian population of 26.51%) as of 2003 of 6500 neonates, revealed 146 cases of HbS carriers (2.4%), 90 cases heterozygous HbC (1.4%), 2 heterozygous HbD (0.02%), and 2 homozygous HbC, without cases of homozygous S10; however, there is no definition of inclusion criteria (especially of gestational age) and of exclusion criteria (transfusions prior to taking the sample). There is no knowledge of the prevalence of the types of abnormal hemoglobins (S, C, D, and G) in newborns in the city of Buenaventura (Valle del Cauca, Colombia). This city, located in the Pacific region, has a population projected for 2005 of 278,960 inhabitants (86% living in the urban zone)11. The proportion of Afro-Colombian population is of 92%. Previous studies of cost-effectiveness analysis favor neonatal detection aimed at, that is, only newborns from some ethnic groups12,13 when the individuals at risk are few among the population; however, universal detection identifies more newborns with the disease and prevents more deaths14,15.
Early detection of homozygous S newborns permits the application of broadly recognized integral programs in health services, vaccines, monitoring, and education for children and their parents2, diminishing the incidence of infection and associated mortailty16. Parent education on disease and above all the symptoms (e.g., splenic sequestration) permits opportune attention of severe cases. The possibility of curing via allogenic stem cell transplants informed in the literature, although still promissory, assigns more relevance to detection17.
The objective of this study was to determine the prevalence of hemoglobins S, C, D, and G in newborns from a high-risk zone like the city of Buenaventura.
METHODS
A descriptive cross-sectional study was conducted. Universal screening was employed15.To estimate the sample size, we used the information on the projected population for the city of Buenaventura for 2005, because the data for the 2005 census and projections for subsequent years had not been published18. Said projection reports 278,960 inhabitants (86% located in the urban zone)11. To estimate the sample size, the StatCalc 6th version program by EpiInfo was used based on the following considerations: estimated prevalence of hemoglobinopathies (S, C, D, G) equal to 10%9, margin of error of 3%, and confidence level of 95%. The representative minimum sample size was of 384 newborns.
The samples were taken from newborns at three hospitalization centers in the city: Departmental Hospital of Buenaventura – complexity level 2, Luis Ablanque de la Plata Hospital – complexity level 1, and the Buenaventura Clinic – complexity level 2 (these are the hospitalization centers in Buenaventura authorized for childbirth attention by the Valle del Cauca Departmental Secretary of Public Health), these were taken after informed signed consent by legal guardians.
Inclusion criteria. Gestational age of 36 or more weeks (corroborated by obstetric sonogram, given that more premature newborns do not express in adequate proportion the hemoglobins of the adult stage19), domiciled in the urban area of Buenaventura, born between 01 February 2007 and 01 February 2008. On an adjoining card, the following data were registered: motherís name (or legal guardian), motherís age (in years), telephone number, home address, gestational age, date of birth, sex (female or male), and weight of the newborn. Exclusion criteria: newborns with red blood cell transfusion prior to taking the sample.
Taking of blood sample and storage. Using a syringe, 1 ml of blood was drawn from the umbilical cord placental end in loop form after its ligation (or from the heel, in case of not having been obtained from the first site or due to bad previous sample taking) and a drop was fixed on each circle of the filter paper (a total of 4 drops), and stored at 4ºC.
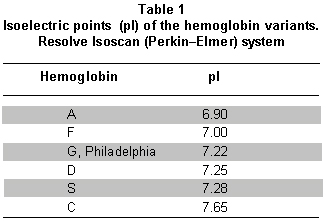
Analytical methods. The isoelectric focusing electrophoresis (IFE) technique was selected because of the low costs of equipment and samples, high sensitivity (100%), and specificity (100%) compared to methods like electrophoresis in cellulose agar and high-performance liquid chromatography20 with low intra- and inter-assay error21, permitting reproduction of the results. With IFE, each hemoglobin has a defined isoelectric point (pI) (Table 1). To develop the methodology, we used the Wallac RESOLVE® Neonatal Hemoglobin test kit (PerkinElmer, 940 Winter Street, Waltham, Massachusetts 02451, USA)21,22.
The results of the hemoglobins were reported in qualitative order according to the percentage of hemoglobins present like: FA (normal), FS (homozygous Hb S), FAS (carrier Hb S), FC (homozygous Hb C), FAC (carrier Hb C), FSC (mixed S and C heterozygous), FAD (carrier Hb D), and FAG (carrier Hg G) (Graph 1).
The statistical analysis was conducted based on percentage distributions, prevalence, and confidence intervals of 95% - according to the exact binomial method using the Epi Info Software for Windows, version 3.3.2.
Ethical considerations. The study was approved by the Institutional Committee on Human Ethics Review of the Faculty of Health at Universidad del Valle. The sample was taken from each newborn after informed signed consent from one of the parents or legal guardian.
RESULTS
In total, 399 samples were collected from newborns at three Institutions: Departmental Hospital of Buenaventura 252 (63.2%), Luis Ablanque de Plata Hospital 113 (28.3%), and Buenaventura Clinic 34 (8.5%). Some 98.7% of the samples were taken from the umbilical cord; the rest were collected from the heel.A total of 50.6% of the newborns were females. The average gestational age was 39.3 ± 1.1 weeks, with average weight at birth of 3114 ± 505 g. The prevalence of newborns with at least one abnormal hemoglobin was of 11.7%, with 8.6%-15.1% having a confidence interval of 95% (Table 2, Graph 2, Graph 3, Graph 4). No significant statistical differences were found regarding gender or the gestational age of the newborns with respect to the hemoglobin pattern.
DISCUSSION
Although the total percentage of abnormal hemoglobins in this study is similar to prior studies7-9, we note a greater proportion of newborns with hemoglobin C, in comparison with previous studies in the zone. Hemoglobin C is more frequent in Western Africa23 and it is associated to minor symptoms related to malaria infection5,24. Homozygous C has lower complications related to sickle-cell anemia when compared to homozygous S. They may even be asymptomatic into adulthood, although the retinopathy related to sickle-cell anemia is more frequent with hemoglobin C25, requiring periodic ophthalmologist control of these individuals.
The previously mentioned studies conducted in adults and children older than one year of age could underestimate the prevalence in the population when not including the children who perished because of sickle-cell disease before reaching one year of life, and not including individuals with hemoglobin C that may not have attended the center of reference for malaria infection (because of manifesting few or no symptoms).
Only one newborn with combined heterozygous SC was found, warranting a periodic monitoring. In these individuals, it was not establish the effectiveness of the antibiotic prophylaxis to diminish mortality due to pneumococcus, which has indeed benefited homozygous SS individuals. The current study found no homozygous S or C, given that the design was not made for individuals with the disease of sickle-cell anemia; this requires a larger sample size. When the screening detects a newborn with hemoglobins FS or FC, the test must be confirmed before two months of age to discard the co-existence of beta+ thalassemia26.
In 2007, 4546 babies were born in the urban zone of Buenaventura27. The study sample of 399 newborns represents 8.7% (399/4546) of the live births for 2007, hence, applying for this period.
The high prevalence of hemoglobin alterations in a high-risk population, like Buenaventura, suggests the need for an universal neonatal detection program in these types of zones. This should be done for the purpose of early detection of homozygous S or C individuals, and opportunely initiate preventive and educational measures tending to diminishing morbidity and mortality related to sickle-cell anemia.
Conflict of interest. None of the authors has conflicts of interest related to this study.
ACKNOWLEDGMENTS
We thank the directors and personnel from the neonatal wards at the Departmental Hospital in Buenaventura, the Luis Ablanque de la Plata Hospital, and the Buenaventura Clinic for their collaboration. We also thank John Jairo Lozano and María del Pilar Silva R, professionals at the Endocrine and Metabolism Laboratory in the Faculty of Health at Universidad del Valle for their collaboration in administrative and budget matters and in the transcription of documents.REFERENCES
1. Weatherall D, Clegg J. Inherited haemoglobin disorders: an increasing global health problem. Bull WHO. 2001; 79: 704-12.
2. American Academy of Pediatrics, Section on Hematology/Oncology, Committee on Genetics. Health supervision for children with Sickle Cell Disease. Pediatrics. 2002; 109: 526-35.
3. Steinberg M. Management of Sickle Cell Disease. N Engl J Med. 1999; 340: 1021-30.
4. Platt O, Brambilla D, Rosse W, Milner P, Castro O, Steinberg M et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994; 330: 1639-44.
5. Agarwal A, Guindo A, Cissoko Y, Taylor J, Coulibaly D, Koné A et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood. 2000; 96: 2358-63.
6. Strahan, J, Canfield M, Drummond-Borg L, Neill S. Ethnic and gender patterns for the five congenital disorders in Texas from 1992 through 1998. Tex Med. 2002; 98: 80-6.
7. Moyano M, Méndez F. Defectos eritrocíticos y densidad de la parasitemia en pacientes con malaria por Plasmodium falciparum en Buenaventura, Colombia. Rev Panam Salud Publica. 2005; 18: 25-32.
8. Pereira F, Sáenz I. Hemoglobinopatías en niños. Colomb Med. 1996; 27: 146-9.
9. Muñoz N, Pereira F, Sáenz I. Hemoglobinas anormales en Salahonda (Tumaco). Acta Pediatr Colomb. 1994; 1: 7-10.
10. Satizábal J, Neuta P, Muñoz J, Somoyar P. Incidencia de hemoglobinopatías en neonatos de Cali. Salud Uninorte. 2004; 18: 71-2.
11. DANE. Proyecciones de población, por área, según municipios 1995-2005. Bogotá, DC: DANE.
12. Panepinto J, Magid D, Rewers M, Lane PA. Universal versus targeted screening for sickle cell disease: a cost effectiveness analysis. J Pediatr. 2000; 136: 201-8.
13. Tsevat, J, Wong, J, Pauker, S, Steinberg, M. Neonatal screening for sickle cell disease: A cost-effectiveness analysis. J Pediatr. 1991; 118: 546-54.
14. Henthorn, JS, Almeida, AM, Davies, SC. Neonatal screening for sickle cell disorders. Br J Haematol. 2004; 124: 259.
15. Consensus Development Panel. National Institutes of Health. Newborn screening for SCD and other hemoglobinopathies. JAMA. 1987; 258: 1205-9.
16. Gaston M, Verter J, Woods G, Pegelow C, Kelleher J, Presbury G et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986; 314: 1593-9.
17. Bernaudin F, Socie G, Kuentz M, Chevret S, Duval M, Bertrand Y et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007; 110: 2749-56.
18. Departamento Administrativo Nacional de Estadística. Síntesis del proceso de cierre del Censo General 2005. Bogotá: DANE; 2008.
19. Telen M. The mature erythrocyte. En: Greer JP, Foerster J, Rodgers, GM, Paraskevas F, Glader B, Arber DA et al. Wintrobeís clinical Hhematology. 12th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 126-55.
20. Sickle Cell Disease Guideline Panel. Sickle Cell Disease: screening, diagnosis, management, and counseling in newborns and infants. Clinical practice guideline N° 6. AHCRP Pub. N° 93-0562. Rockville: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; 1993.
21. Hempe J, Craver R. Quantification of hemoglobin variants by capillary isoelectric focusing. Clin Chem. 1994; 40: 2288-05.
22. Basset P, Beuzard Y, Garel M, Rosa J. Isoelectric focusing of human hemoglobin: its application to screening, to the characterization of 70 variants, and to the study of modified fractions of normal hemoglobins. Blood. 1978; 51: 971-82.
23. Devoucoux R, Hurpin C, Baudon D, Molez J, Roux J, Guilloud-Bataille M et al. Population genetics of abnormal haemoglobins in Burkina Faso, west Africa. Ann Hum Biol. 1991; 18: 295-302.
24. Rihet P, Flori L, Tall F, Traoré A, Fumoux F. Hemoglobin C is associated with reduced Plasmodium falciparum parasitemia and low risk of mild malaria attack. Hum Mol Genet. 2004; 13: 1-6.
25. Condon P, Gray R, Serjeant G. Ocular findings in children with sickle cell haemoglobin C disease in Jamaica. Br J Ophthalmol. 1974; 58: 644-9.
26. Strickland D, Ware R, Kinney T. Pitfalls in newborn hemoglobinopathy screening: Failure to detect ß+-thalassemia. J Pediatr. 1995; 127: 304-8.
27. Departamento Administrativo Nacional de Estadística. Cuadro 7. Nacidos vivos por sitio del parto, según departamento, municipio y área de ocurrencia. Nacimientos 2007. URL disponible en: http://www.dane.gov.co/files/investigaciones/poblacion/nacimientos/nac_07/Cuadro7.xls