Evidence
in Colombia of 625G>A polymorphism in the short chain acyl-CoA
dehydrogenase gene, a variation which could cause glutaric aciduria in
our populations
José Henry Osorio, PhD*
* Department of Basic Sciences for Health, Research Laboratory in
Clinical Biochemistry and Molecular Pathology, Universidad de Caldas,
Manizales, Colombia. e-mail: jose.osorio_o@ucaldas.edu.co
Received for publication June 12, 2009 Accepted for publication March 2, 2010
SUMMARY
Introduction:
Short-chain acyl-CoA dehydrogenase (SCAD) is a homotetrameric
mitochondrial flavoenzyme that catalyzes the initial reaction in
short-chain fatty acid b-oxidation. The SCAD gene is located on
chromosome 12q22 and is approximately 13 kb long with 10 exons and 1236
nucleotides of coding sequence. Hereditary SCAD deficiency has been
reported and only a few cases of this disorder have been described.
Objective:
The present study was conducted to determine the possible presence of
the 625G>A variation in the short-chain acyl-CoA dehydrogenase gene
in Caldas (Colombia), given that variations 625G>A and 511C>T are
present in 14% of some studied populations; thereby sometimes causing
its deficiency.
Methods:
This is a descriptive study; blood samples from three-hundred adult
volunteers were tested for 625G>A polymorphism, analysing the
polymerase chain reaction amplified cDNA, using a single-stranded
conformation polymorphism assay. The results were confirmed by direct
bidirectional cycle sequencing using DNA from the positive persons.
Results: The polymorphism was identified and confirmed in four healthy persons.
Conclusion: This is
evidence of the presence of 625G>A polymorphism in the short-chain
acyl-CoA dehydrogenase gene in Colombia, meaning that some people in
our populations can be at risk of suffering SCAD deficiency and its
main complication: the ethylmalonic aciduria.
Keywords: Short-chain acyl-CoA dehydrogenase; 625G>A polymorphism; Ethylmalonic aciduria; b-oxidation.
Evidencia del polimorfismo
625G>A en el gen de la acil-CoA deshidrogenasa de cadena corta en
Colombia, una variación que podría causar aciduria
glutárica en algunas poblaciones del país
RESUMEN
Introducción: La
acil-CoA deshidrogenasa de cadena corta (SCAD) es una flavoenzima
homotetramérica mitocondrial que cataliza la reacción
inicial de la â-oxidación de los ácidos grasos de
cadena corta. El gen SCAD se ubica en el cromosoma 12q22, con una
longitud de 13 kb, con 10 exones y 1236 nucleótidos de secuencia
codificadora. Se ha informado la deficiencia hereditaria de
SCAD y se han descrito pocos casos de la deficiencia.
Objetivo: El presente
estudio buscó determinar la posible presencia del polimorfismo
625G>A en Caldas, Colombia, debido a que las variantes 625G>A y
511C>T en el gen de la acil-CoA deshidrogenasa de cadena corta
están presentes en 14% de algunas poblaciones estudiadas,
causando algunas veces su deficiencia.
Métodos: El
presente estudio es descriptivo; se estudiaron muestras de sangre de
300 voluntarios para el polimorfismo 625G>A mediante la
técnica de polimorfismo de conformación de la cadena
simple, con ADN amplificado por reacción en cadena de la
polimerasa. Los resultados se confirmaron por
secuenciación.
Resultados: El polimorfismo se identificó en cuatro personas aparentemente sanas.
Conclusión:
Existe evidencia de la presencia del polimorfismo 625 G>A en el gen
de la acil-CoA en Colombia, lo que significa que algunas personas en
las poblaciones del país pueden estar en riesgo de sufrir
deficiencia de SCAD y su principal complicación, la aciduria
etilmalónica.
Palabras claves: Acil-CoA deshidrogenasa de cadena corta; Polimorfismo 625G>A; Aciduria etilmalónica; b-oxidación.
All the currently identified mitochondrial fatty acid (FA) b-oxidation
defects are autosomal recessive. The clinical manifestations result
from the inability of FA-oxidising tissues to keep up with increased
energy demands; therefore, target organs of FA oxidation defects
include skeletal and cardiac muscles in addition to the liver1.
Hypoketotic hypoglycaemia is present in nearly all the defects, and
usually occurs following an intercurrent illness, but may occasionally
be seen after a short fast. In general, FA oxidation disorders should
always be included in the differential diagnosis of unexplained
hypoglycaemia, metabolic acidosis, Reye’s like syndrome,
myopathy, recurrent myoglobinuria, and cardiomyopathy2.
Laboratory findings reinforce the clinical diagnosis, levels of
intermediary metabolites in urine (glucose, ketone bodies, lactate,
pyruvate), and blood (non-sterified FA)3; urinary organic acid profile4; body fluids and tissue acylcarnitine analysis5; enzyme measurement and pathway intermediates in cultured cells and leukocytes6,7, and DNA analysis8 are used to confirm the diagnosis of any alteration.
Short-chain acyl-CoA dehydrogenase deficiency is a poorly characterized
mitochondrial fatty acid b-oxidation disorder with a very variable
clinical picture and at least 35 inactivating mutations and some
polymorphic variants have been reported in the SCAD gene9. Hereditary SCAD deficiency was first reported in 198410 and only few cases of this disorder have been described for either classic SCAD deficiency or variant SCAD10-13.
A clinically reliable diagnosis requires either a muscle biopsy for the
measurement of the enzymatic SCAD activity or molecular genetic
analysis of the SCAD gene, both only available in research
laboratories. This situation is further complicated by the presence of
the mainly two variants (625 G>A and 511C>T) in the SCAD gene
that are frequent in the European population, and have been reported to
confer disease susceptibility14. In the 625G>A variation
the polymorphic site is a transition from G to A at position 625 (A625)
of the coding region of the cDNA, changing a glycine to serine at amino
acid position (G185S) of the precursor protein. The variant 625G>A
has been shown to be associated with ethymalonic aciduria and other
biochemical findings15. Because urinary ethymalonic acid (EMA) elevation most likely reflects a cellular accumulation of butyryl-CoA16,
which is secondary to reduced SCAD catalytic activity, these patients
are correctly considered as possibly having SCAD deficiency.
Despite the fact that most patients with EMA aciduria have not had
their SCAD activity determined, an association between elevated EMA and
SCAD has been documented by the presence of either or both of two SCAD
gene susceptibility variations in 69% of patients with EMA aciduria17.
The 625G>A variant shows homozygous prevalence of 60%, as SCAD
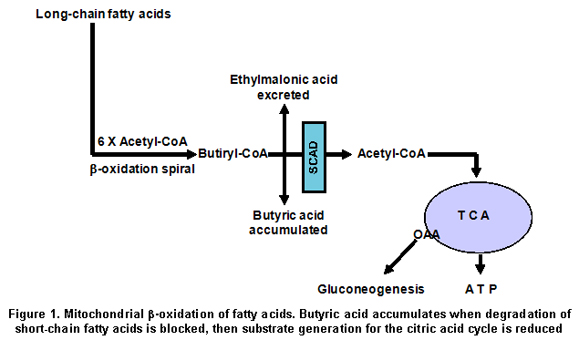
protein presents reduced stability compared to the control SCAD protein18. As SCAD is a key enzyme in the oxidation of fatty acids, which serve as substrates for the gluconeogenesis (Figure 1),
reduced SCAD activity because of the 625G>A variant for fasting
hypoglycaemia, and ethymalonic aciduria as shown in the SCAD
deficiency. The present study was conducted to determine the possible
presence of the 625G>A variation in the short-chain acyl-CoA
dehydrogenase gene in Caldas, Colombia, as the 625G>A and 511C>T
variations are present in 14% of some populations studied, sometimes
causing its deficiency.
MATERIALS AND METHODS
The present study is descriptive. Blood samples used in this study were
from 300 adult, healthy volunteers between 18 and 49 years of age (120
men and 180 women), all born in Caldas, Colombia, apparently none
suffering from any inherited inborn error of metabolism. According to
the number of persons visiting our laboratory, samples were collected
between January 2006 and January 2009, which means that samples were
obtained by convenience, and all participants signed written consent.
Blood samples were extracted in tubes containing EDTA. DNA extraction was performed according to Gustafson et al.19,
with some modifications. The polymorphism was identified by comparing
the polymerase chain reaction (PCR) and amplified cDNA from a
previously studied person carrying the polymorphism with the apparently
healthy volunteers. A single-stranded conformation polymorphism (SSCP)
assay, based on the following primers was used: Sense primer:
5’-GCAGCTCTGAGAAAACCAC. Antisense primer: 5’-ATGTCC
AGGGTTTGCTGT. PCR Conditions: 3 min at 94°C, 40 sec at 94°C, 30
sec at 55°C, and 2 min at 72°C for 35 cycles with 500 ng of
purified genomic DNA as template and 75 ng of each primer. When DNA
fragments were subjected to electrophoresis in 8%
acrilamide/bisacrilamide (19:1), 7.5 M urea gel, at room temperature
for 3 hours, the single-base change at position 625 was clearly
detected after silver stain. The results were confirmed by direct
bidirectional cycle sequencing using DNA.
According to Article 11 on its literal a from Resolution N° 8430
promulgated by the Ministry of Health for Scientific, technical and
administrative guidelines for health research, the present study is
considered without risk. The study was approved by the corresponding
ethics committee.
RESULTS
After analyzing DNA samples from the 300 participants, the polymorphism
was identified in four apparently healthy adult volunteers -all in
heterozygosis. The first case was a 21-year-old male who was born from
non-consanguineous parents, another two individuals were identified as
carriers of the polymorphism; they are the parents of a family who
presented two cases of sudden infant death after two consecutive
pregnancies and they are cousins. After a third pregnancy, a baby was
born and after 2 years she is still alive without problems; she did not
present the 625G>A polymorphism. A fourth person was identified as
carrying the polymorphism, an apparently normal 22-year-old woman. The
four persons are from families without antecedents of any disease that
could be related to some fatty acid oxidation disorder; the
acylcarnitine analysis was normal for all of them.
DISCUSSION
Colombia is divided into 35 regions with an approximate total
population of 45-million inhabitants, presenting racial mixture. Data
obtained from the 2005 census shows that Caldas (a Colombian region)
has a population of 968,740 inhabitants distributed within 29
municipalities20. For us, it is very important to detect the
presence of this polymorphism to demonstrate the presence of
fatty-acid-oxidation inherited diseases among us, and it is a valuable
contribution for the future understanding of geographical distribution
and ethnic origin elucidation of SCAD deficiency, as no previous
reports have been published showing the presence of this polymorphism
in these countries, while being mainly reported in Europe and the
United States21. The first person presenting this variant in
our study has a very interesting genetic charge for these kinds of
works, as his father is from Tumaco, a region of black population and
his mother is from the north of Antioquia, a place characterized by the
presence of a strong Spaniard white genotype. The other 3 persons are
from the centre of the country.
There are reports of ethnic differences observed with respect to the
625G>A variant, while the allele frequency was similar between
Caucasians and Hispanics (25 and 30%, respectively), African-Americans
and Asians carried the 625G>A variant less frequently (9 and 13%,
respectively)22. Corydon et al.14, found the 625A
variant allele in homozygous form in 60% of 135 patients with elevated
EMA excretion, analysed because a suspicion of a metabolic disorder,
compared with 7% occurrence in the general population. That is why,
since about 10-14% of the general population is homozygous 625G>A or
511C>T or compound heterozygous for both, it is necessary to have
some other indication that they are disease-associated. It is generally
accepted that some biochemical analysis can be performed to confirm if
the person carrying the polymorphism is suffering the disease. Because
these patients are phenotypically diverse, the analysis of blood
acylcarnitines is a good tool for diagnosing SCAD deficiency. The
acylcarnitine profile in these patients is characterized by high blood
concentrations of butyrylcarnitine (C4-acylcarnitine). The
acylcarnitine profile for our patients was normal; hence, they are
carrying the polymorphism without suffering the disease.
CONCLUSION
Short-chain acyl-CoA dehydrogenase (SCAD) deficiency is a clinically
heterogeneous disorder. The clinical phenotype varies from fatal
metabolic decompensation in early life to subtle adult onset, and some
patients remain asymptomatic. Two mutations (511C>T; 625G>A) have
been described in exons 5 and 6 of the SCAD gene, respectively. These
variants are not true disease-causing mutations but can confer disease
susceptibility because they alter the structural and catalytic
properties of the SCAD protein. Although the prevalent finding for SCAD
deficiency is neurological abnormalities, such as hypotonia and
seizures accompanying general developmental delay, the population of
patients with ethylmalonic aciduria with a high frequency of the 625A
variant allele in the homozygous form also showed diverse phenotypes,
typically neuromuscular symptoms, and hypoglycaemia. The acylcarnitine
analysis is an important tool in diagnosing the disease, given that a
high concentration of C4-acylcarnitine is consistent with a biochemical
diagnosis of SCAD deficiency. This kind of study is important for us
because it shows the presence of mutations or polymorphisms, which can
cause some metabolic alterations related to enzymatic dysfunction of
some biochemical ways, not reported or treated among us. We need to
continue studying our populations to know the real frequency of this
polymorphism because the principal studies have been performed mainly
in Caucasoid populations.
Conflict of interest. None of the author had conflicts of interest related to this study.
REFERENCES
1. Stanley A. Carnitine disorders. Adv Pediatr. 1995; 42: 209-42.
2. Lteif AN, Schwenk WF. Hypoglycemia in infants and children. Endocr Metab Clin North Am. 1999; 23: 619-48.
3. Moser HW, Moser AB. Measurement of saturated very long chain fatty
acids in plasma. In: Hommes FA (ed.). Techniques in diagnostic human
biochemical genetics: a laboratory manual. New York: Wiley-Liss; 1991.
p.177-92.
4. Greter J, Jacobson CE. Urinary organic acids: isolation and
quantification for routine metabolic screening. Clin Chem. 1987; 33:
473-80.
5. Millington DS, Kodo N, Norwood DL, Roe CR. Tandem Mass Spectrometry:
A new method for acylcarnitine profiling with potential for neonatal
screening for inborn errors of metabolism. J Inher Metab Dis. 1990; 13:
321-4.
6. Pourfarzam M, Schaefer J, Turnbull DM, Bartlett K. Analysis of fatty
acid oxidation intermediates in cultured fibroblasts to detect
Mitochondrial oxidation disorders. Clin Chem. 1994; 40: 2267-75.
7. Schaefer J, Pourfarzam M, Bartlett K, Jackson S, Turnbull DM. Fatty
acid oxidation in peripheral blood cells: characterisation and use for
the diagnosis of fatty acid oxidation. Pediatr Res. 1995; 37: 345-60.
8. Ziadeh R, Hoffman EP, Finegold DN. Medium chain Acyl-CoA
dehydrogenase deficiency in Pennsylvania: neonatal screening shows high
incidence and unexpected mutation frequencies. Pediatr Res. 1995; 37:
675-8.
9. Jethva R, Bennett MJ, Vockley J. Short-chain acyl-coenzyme A dehydrogenase deficiency. Mol Gen Metab. 2008; 95: 195-200.
10. Turnbull DM, Bartlett K, Stevens DL, Alberti KGMM, Gibson GJ,
Johnson MA, et al. Short-chain acyl-CoA dehydrogenase deficiency
associated with a lipid-storage myophaty and secondary carnitine
deficiency. N Engl J Med. 1984; 311: 1232-36.
11. Amendt BA, Greene C, Sweetman L, Cloherty J, Shih V, Moon A, et al.
Short-chain acyl-coenzyme A dehydrogenase deficiency. Clinical and
biochemical studies in two patients. J Clin Invest. 1987; 79: 1303-9.
12. Coates PM, Hale DE, Finocchiaro G, Tanaka K, Winter SC. Genetic
deficiency of short-chain acyl-conzyme A dehydrogenase in cultured
fibroblasts from a patient with muscle carnitine deficiency and severe
skeletal muscle weakness. J Clin Invest. 1988; 81: 171-5.
13. Baerlocher KE, Steinmann B, Aguzzi A, Krähenbühl S, Roe
CR, Vianey-Saban C. Short-chain acyl-CoA dehydrogenase deficiency in a
16-year-old girl with severe muscle wasting and scoliosis. J Inherit
Metab Dis. 1997; 20: 427-31.
14. Corydon MJ, Gregersen N, Lehnert W, Ribes A, Rinaldo P, Kmoch S.
Ethylmalonic aciduria is associated with an amino acid variant of
short-chain acyl-coenzyme A dehydrogenase. Pediatr Res. 1996; 39:
1059-66.
15. Burlina AB, Dionisi-Vici C, Bennett MJ, Gibson KM, Servidei S,
Bertini E, et al. A new syndrome with ethylmalonic aciduria and normal
fatty acid oxidation in fibroblasts. J Pediatr. 1994; 124: 79-86.
16. Hegre CS, Halenz DR, Lane MD. The enzymatic carboxilation of butyryl-coenzyme A. J Am Chem Soc. 1959; 81: 6526-7.
17. Moczulski D, Majak I, Mamczur D. An overview of beta-oxidation disorders. Postepy Hig Med Dosw. (Online) 2009; 8: 266-77.
18. van Maldegem BT, Durán M, Wanders RJ, Niezen-Koning KE,
Hogeveen M, Ijlst L, et al. Short-chain acyl-CoA dehydrogenase
deficiency (SCADD): relatively high prevalence in the Netherlands and
strongly variable fenotype; neonatal screening not indicated. Ned
Tijdschr Geneeskd. 2008; 26: 1678-85.
19. Gustafson S, Proper JA, Bowie EJW, Sommer SS. Parameters affecting
the yield of DNA from human blood. Biochemistry. 1987; 165: 294-9.
20. Departamento Nacional de Estadística (DANE). Colombia Censo
General 2005. [fecha de acceso: enero 20 de 2009 ]. URL disponible en: http:// www.dane.gov.co/censo/
21. Gregersen N, Andresen BS, Corydon MJ, Corydon TJ, Olsen RK, Bolund
L, et al. Mutation analysis in mitochondrial fatty acid oxidation
defects: Exemplified by acyl-CoA dehydrogenase deficiencies, with
special focus on genotype-phenotype relationship. Hum Mutat. 2001; 18:
169-89.
22. Corydon MJ, Vockley J, Rinaldo P, Rhead WJ, Kjeldsen M, Winter V,
et al. Role of common gene variations in the molecular pathogenesis of
short-chain acyl-CoA dehydrogenase deficiency. Pediatr Res. 2001; 49:
18-23.
|