Allelic polymorphism in the serotonin transporter gene in major depression patients*
Carlos H. Escobar, MSc1, Jorge Hernán Calderón, MSc2, Germán Alberto Moreno, MSc3
* Funding: Dirección Territorial de Salud de Caldas, Universidad de Caldas and Universidad Tecnológica de Pereira, Colombia.
1. Professor, CBS Group, Fundación Universitaria de Ciencias de la Salud, Bogotá, DC, Colombia. e-mail: chescobar@fucsalud.edu.co
2. Professor, Department of Mental Health and Human Behavior, Universidad de Caldas, Manizales, Colombia. e-mail: calonva@yahoo.com
3. Professor, Department of Community Medicine, Universidad Tecnológica de Pereira, Pereira, Colombia. e-mail: gamo@telmex.net.co
Received for publication January 21, 2009 Accepted for publication December 21, 2010
SUMMARY
Introduction: Major depression (MD), like other mood disorders, is considered a worldwide endemic pathology, becoming one of the biggest public health problems. The hereditary factors of mood disorders have been studied for many years and different chromosomal regions and genes have been involved in this physiopathological process.
Objective: To establish the association between allelic variants of the serotonin transporter gene (5-HTTLPR and VNTR) and MD in a population with this diagnostic in the department of Caldas, Colombia.
Materials and methods: A case-control study was conducted with individuals older than 16 years of age born in the department of Caldas. The sample was composed of 59 patients with the MD with family antecedents of the pathology and 59 controls paired by precedence, age, and gender. For the cases and controls selection the Diagnostic Interview for Genetic Studies (DIGS) was used. Using the Hranilovic et al. protocol, the polymorphic regions in the promoter and third intron of the Serotonin Transporter gene was amplified.
Results: It was not possible to find association between MD and the genetic or clinical variables. The absence of the short allele of the promoter could act as a protective factor (OR=0.70 CI 95%=0.313 to 1.604), for the development of the pathology in this population, and the presence of at least one copy of the 10 repetition alleles of the third intron could act as a risk factor (OR: 1.25), but the wide confidence interval (CI 95%=0.38 to 2.64) does not permit supporting these conclusions.
Discussion: The results obtained in this population do not yield conclusive information related with the etiopathogeny of MD, but do not contradict those obtained in other studies with bigger samples than ours. The broad confidence interval does not support conclusions about the role as a risk factor for the 10 repetition alleles of the intron or the absence of the S allele of the promoter as a protector factor. Further studies with larger population samples may help to clarify these facts.
Keywords: Major depression; 5-HTTLPR; VNTR; Colombia.
Colomb Med. 2011; 42: 48-53
Polimorfismos alélicos del gen del transportador de serotonina en pacientes con depresión mayor
RESUMEN
Introducción: La depresión mayor (DM), como los demás trastornos del estado de ánimo, es considerada un síndrome endémico en el contexto mundial. Por ello se ha constituido en uno de los más grandes problemas de salud pública. Los factores hereditarios de los trastornos del estado de ánimo han sido estudiados por muchos años y en su proceso fisiopatológico se han involucrado diferentes porciones cromosómicas y genes.
Objetivo: Establecer la asociación entre las variantes alélicas del gen del transportador de serotonina (5-HTTLPR y VNTR) y la DM, en una población con este diagnóstico en el departamento de Caldas, Colombia.
Materiales y métodos: Se realizó un estudio de casos y controles en personas mayores de 16 años originarias del departamento de Caldas. La muestra incluyó 59 pacientes con el síndrome y antecedentes familiares de DM y 59 sujetos controles pareados por procedencia, edad y género. Para la selección de los casos y controles se utilizó el instrumento Diagnostic Interview for Genetic Studies (DIGS). Se usó el protocolo de Hranilovic et al., se amplificaron las porciones polimórficas del promotor y el tercer intrón del gen del transportador de serotonina.
Resultados: No se encontró asociación entre la DM y las variables clínicas o genéticas. La ausencia del alelo corto del promotor podría actuar como factor protector (OR=0.70 IC 95%= 0.313 a 1.604), para el desarrollo de este síndrome en la población colombiana y la presencia de al menos una copia del alelo de 10 repeticiones del tercer intrón, podría actuar como factor de riesgo (OR: 1,25), pero el amplio intervalo de confianza (IC 95%=0.38 a 2.64) no otorga soporte para estas afirmaciones.
Discusión: Los resultados obtenidos con la presente población no entregan información concluyente relacionada con la etiopatogenia de la DM, pero tampoco riñen con los obtenidos en estudios similares con muestras de mayor tamaño. La amplitud de los intervalos de confianza no permiten emitir conclusiones definitivas acerca de factores de riesgo (alelo 10 del intrón) o protectores (ausencia del alelo S del promotor). Se requieren estudios con muestras más grandes que otorguen mayor precisión y poder a estos resultados.
Palabras clave: Depresión mayor; 5-HTTLPR; VNTR; Colombia.
Colomb Med. 2011; 42: 48-53
Major depression (MD), like other mood disorders, is considered a worldwide endemic pathology. There are over 121-million people affected around the world, becoming one of the biggest public health problems. MD is by itself an important risk factor for the development and death caused by cardiovascular disease. It has been shown that as an independent variable, it increases the mortality risk in the same manner as a cerebrovascular accident or a case of congestive heart failure1.
Genetic predisposition is one of the points where most attention has centered. The hereditary factors of MD is recognized by family studies of twins and adopted families2,3; but it is also clear that environmental factors have a clear and critical impact on the development of the pathology; in fact, only some individuals with a family history of MD ever develop it4.
It is currently accepted that as with other psychiatric illnesses, MD is a polygenic disorder5, although mutations have been identified that dramatically increase the possibility of developing the disorder in different contexts6,7.
The serotonin transporter gene is one of the most plausible candidates to being involved in the physiopathological process of mood disorders, but although efforts are being made since the 1990s, results available worldwide are not conclusive8-10.
At least three sources of variability in this gene have been identified, of which the most studied are the promoter polymorphous portion and that of the third intron. Both portions come about because of a variable number of tandem repetitions of an imperfect block. For the promoter, the block repeated has 22 or 23 pairs of bases and has been repeated between 16 and 22 times. The most frequently found variants are of 14 repetitions (short allele, S) and 16 repetitions (long allele, L). The block repeated in the intron, has 17 pairs of bases, found between 9 and 12 times. The most frequently found alleles are those with 10 and 12 repetitions.
In the current work, we studied the possible association among the promoter polymorphisms and the Transporter third intron and the clinical behavior of the major depressive condition in a population with this diagnosis and a control group in the department of Caldas, Colombia.
MATERIALS AND METHODS
We conducted a case and control study in individuals over 16 years of age originating from the department of Caldas in Colombia. The work was divided into two methodological components (clinical and molecular); for the clinical component, we reviewed the clinical charts of personal consultation of psychiatrists and from participating institutions, selecting individuals over 16 years of age originating from the department of Caldas with diagnosis of MD according to DSM IV and CIE-10 criteria. The control for each case was selected according to origin, age, and gender.
For the purpose of selecting the cases and controls, the Diagnostic Interview for Genetic Studies (DIGS) was used, with prior standardization to be administered by the research group. Upon evaluating the level of agreement, two psychiatrists from the work group were selected to evaluate the DIGS. One-hundred patients with a history of major depressive disorder were evaluated, of which 59 fulfilled the inclusion criteria. All the individuals included in the current study signed an informed consent form endorsed by the bioethical committees in the institutions involved. Identities of the subjects and their samples were only known by the person who processed them, who assigned each subject a code for their monitoring within the molecular component of the study.
Genotyping. Using the protocol proposed by Hranilovic et al.11, the polymorphous portions of the promoter and the third intron of the serotonin transporter gene were amplified. Total DNA was extracted from each individual from 5 ml of peripheral blood, using a commercially available kit (Promega).
To amplify via polymerase chain reaction (PCR) a total volume of 20 µl was produced with both primers at a concentration of 0.4 mM, 2.5 mM from each nucleotide, 200 ng of DNA, 0.2 µl of the Go Taq polymerase cocktail (Promega) and 4 µl enzyme buffer.
For amplification of the promoter, we began with initial denaturation for a 2-minute period to go on to 35 cycles of 94°C, 61°C, and 72°C, for one minute in each temperature, to end with a 7-minute final extension cycle. The intron amplification program had 2-minutes of initial denaturation, followed by 45 cycles of 94°C (30 seconds), 53°C (30 seconds), and 72°C (45 seconds), after which a final extension step was developed for a 5-minute period. For results tabulation, we designed an Excel data base and the statistical analysis was carried out in the SPSS version 14 statistical package via univariate and bivariate analysis calculating the odds ratio (OR) for association of variables of interest. Molecular results were crossed with some clinical variables extracted from the interview instrument.
RESULTS
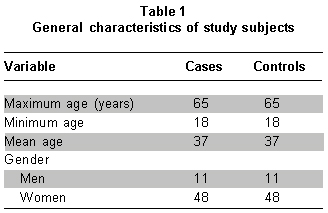
Of the 100 patients with clinical history of MD, 59 fulfilled the inclusion criteria and each was assigned a control maintaining parity between both groups (Table 1). The population analyzed was made up mostly of women (81.4%), the mean age in the cases and controls was 37 years of age. High school education was the leading level of education and nearly 40% of the cases reported having had suicide attempts. The general characteristics of the population analyzed are shown in Table 1.
Three promoter genotypes were identified, SS (24.6%), SL (48.3%), and LL (27.1%). The three genotypes found were distributed within the expected range, per results obtained in similar populations (Table 2)12. Although similar values are presented, the genotype frequencies of the case group reveal some differences with the control group. At least one S allele (S/L=45.8%) was presented in 76.3% of the cases, while 69.5% of the controls had the same characteristic (S/L=50.8%).
Grouping all the individuals with at least one promoter short allele did not generate different results to those found previously (Χ2: 0.203 and 0.352) (Table 3). Hence, the lack of a short allele could act as a protective factor, but our broad confidence interval does not permit emitting a definite conclusion (OR=0.7086, CI 95%= 0.313 to 1.604).
Two alleles were identified in this population for the third intron of the serotonin transporter, of the four described worldwide. The 10/10 genotype had a frequency of 16.9%; 10/12, 46.6%; and the 12/12 had a 36.4% frequency (Table 4). These values keep to the same tendencies expected for this population.
It was noted that 66.1% of the cases had at least one intron 10 allele of (10/12=50.8%), against 59.7% of the controls (10/12=42.4%). Upon evaluating the association between the clinical condition and the allelic variants of the third intron, we found that the 10-repetition allele could be considered a risk factor; but the broad confidence interval does not permit emitting a definite concept (OR=1.246 CI 95%=0.3832 to 2.6406).
No statistically significant dependence relation was found between the third intron genotypes identified and any of the clinical variables evaluated (suicide attempt, age of illness onset, association with triggering factors).
Nine haplotypes were found and their frequencies varied between 1.7% and 28.8%. The SL/1012 haplotype was most commonly found at a frequency of 28.8% among the case groups and 20.3% among the control groups; while the SL/1010 haplotype was the most infrequent among the case groups (3.4%) and the SS/1010 haplotype was least found among control groups (1.7%). No relationship was found between specific haplotypes and the development of depressive symptoms (Χ2: 0.496) (Table 5).
DISCUSSION
The best reference for the current results is that obtained from the study conducted by Ospina-Duque et al.12, which studied a population in Antioquia, Colombia. The frequency of the L/L genotype in our control group (30.5%) is higher than that obtained in the study mentioned (19.6%); while the case group presents b similarity between both studies. The S/S genotype in this work was found less frequently within the control group (18.7%) than that reported by the work carried out in Antioquia (28.2%). But this tendency is not kept in the case group (30.5% reported here and 26.2% reported in the work by Ospina-Duque et al.12). Table 6 shows a detailed comparison of the results obtained by Ospina-Duque et al.12 as well as those obtained in our study.
Upon calculating the odds ratio, no association was found between the promoter allelic variants and the development of depressive conditions. It was not possible to show a statistically significant association among the promoter genotypes and the variables of suicide attempt, age of illness onset, and possible triggering factors like alcohol and illegal psychoactive drugs.
The work presented in 2003 by Caspi et al.8 and others confirming these results13,14 established the idea that the impact of the short allele of this gene's promoter, lies at least partly, on the response to emotional stimuli like child abuse and poor satisfaction of basic needs during childhood. Seemingly, the presence of at least one of the short alleles from the promoter bears functional impact, acting as an incomplete dominance factor, for which it has been proposed that this is a «sensitivity» factor that increases the deleterious emotional impact of the stressful events15,16.
Worldwide findings show that 10- and 12-repetition alleles of the intron, although in different proportions, are the most frequent; situation presented in this study9. The frequencies for each genotype present some variability from one population to another. In the Japanese population, the 10/10 genotype has a 0.5% frequency, the 12/12 genotype has a frequency close to 80%, and the heterozygote is present in nearly 20% of the population9. In Caucasian populations, a lower frequency has been found for the 12/12 genotype (~60%), in addition to a very superior frequency to that found in other populations for the 9-repetition allele (lacking in our work and in the Japanese population)17. The European and American populations present greater frequency in the 10/10 genotype than the rest (~15%), while the other two genotypes have similar frequencies amongst themselves (~45% for 10/12 and ~40% for 12/12)9.
The results obtained through the analysis of the present population do not furnish conclusive information related to the etiopathogenesis of MD; but they do not contradict those obtained in very similar studies with greater-size samples. The current evidence reveals the existence of at least a tangential relationship of the serotonin transporter polymorphisms and the clinical pattern of mood disorders. A physiopathological model is accepted based on diminishing the transcriptional rate the type-S promoter would have18, which has been shown experimentally19. Diminishing the concentration of serotonin in the cleft has been associated with hyperactivity of the amygdale, an essential element for the functional regulation of the limbic system.
These findings generate a series of new research questions. The size of the confidence interval of our work did not permit issuing definite conclusions about the risk factors (10 repetition allele of the intron) or factors like lack of promoter S allele, but it seems plausible that they exist in our reference population. For future studies, it would be interesting to bear in mind some other clinical variables of the disorder, particularly the response to antidepressants and the long-term evolution with bigger-size samples that permit greater precision and greater power of definition of the results.
Conflict of interest. None of the authors has conflicts of interest related to this study.
REFERENCES
1. Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000; 160: 1761-8.
2. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. Am J Psychiatry. 2000; 157: 1552-62.
3. Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006; 163: 109-14.
4. Milne BJ, Caspi A, Harrington H, Poulton R, Rutter M, Moffitt TE. Predictive value of family history on severity of illness: the case for depression, anxiety, alcohol dependence, and drug dependence. Arch Gen Psychiatry. 2009; 66: 738-47.
5. Mill J, Petronis A. Molecular studies of major depressive disorder: the epigenetic perspective. Mol Psychiatry. 2007; 12: 799-814.
6. Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, et al. Loss of function mutation in Tryptophan Hydroxilase-2 identified in unipolar depression. Neuron. 2005; 45: 11-6.
7. Hill K, Sahhar M. Genetic counseling for psychiatric disorders. MJA. 2006; 185: 507-10.
8. Caspi A, Suqden K, Moffitt TE, Taylor A, Craig IW, Harringnton H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003; 31: 386-9.
9. Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes enconding for serotonin receptors and the serotonin transporter: I. Affective disorders. Mol Psychiatry. 2003; 8: 574-91.
10. Gotlib I, Joorman J, Minor K, Hallmayer J. HPA-Axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008: 63: 847-51.
11. Hranilovic D, Schwab SG, Jernej B, Knapp M, Lerer B, Albus M, et al. Serotonin transporter gene and schizophrenia: evidence for association/linkage disequilibrium in families with affected siblings. Mol Psichiatry. 2000; 5: 91-5.
12. Ospina-Duque J, Duque C, Carvajal-Carmona L, Ortiz-Barrientos D, Soto I, Cuartas M, et al. An association study of bipolar mood disorder (type I) with the 5-HTTLPR serotonin transporter polymorphism in human population isolate form Colombia. Neurosci Lett. 2000; 292: 199-202.
13. Kaufman J, Yang BZ, Douglas-Palumberi H, Hourshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA. 2004; 101: 17316-21.
14. Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006; 163: 1588-93.
15. Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressfull life events and a serotonin transporter polymorphism in the prediction of episodes of major depression. Arch Gen Psychiatry. 2005; 62: 529-35.
16. Gotlib I, Joorman J, Minor K, Hallmayer J. HPA-Axis Reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008: 63: 847-51.
17. Mergen H, Karaaslan C, Mergen M, Deniz Ozsoy E, Ozata M. LEPR, ADBR, IRS-1, and 5-HTT gene polymorphisms do not associate with obesity. Endocrine J. 2007; 1: 89-94.
18. Hariri A, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006; 10: 182-91.
19. Smith GS, Lotrich FE, Malhorta AK, Lee AT, Ma Y, Kramer E, et al. Effects of serotonin transporter promoter polymorphisms on serotonin function. Neuropsycopharmacoly. 2006; 29: 2226-34.