Bioavalaibility and pharmacokinetic comparison of two formulations of metformin 850 mg tablets in healthy Colombian volunteers
Gloria Holguín, Esp1, Fanny Cuesta, IQ2, Rosendo Archbold, MSc3, Margarita Restrepo, MSc4, Sergio Parra, MSc5, Lina Peña, Esp6, Blanca Montoya, Bact7, Juan Carlos Ríos, MSc8, Victoria Eugenia Toro, QF9, Adriana Ruiz, PhD10
1. Full Professor, Department of Pharmacy, Faculty of Pharmaceutical Chemistry, Universidad de Antioquia, Medellín, Colombia. e-mail: gholguin@quimbaya.udea.edu.co
2. Full Professor (ret), ad Honorem, Department of Pharmacology and Toxicology, Faculty of Medicine, Universidad de Antioquia. e-mail: fcuesta@quimbaya.udea.edu.co
3. Full Professor and Director of the Graduate Program, Faculty of Pharmaceutical Chemistry, Universidad de Antioquia, Medellín, Colombia. e-mail: rarchbold@farmacia.udea.edu.co
4. Full Professor, Department of Pharmacy, Faculty of Pharmaceutical Chemistry, Universidad de Antioquia. Chief of the Research Center, Faculty of Odontology, Universidad de Antioquia, Medellín, Colombia. e-mail: mrestrep@farmacia.udea.edu.co
5. Assistant Professor, Department of Pharmacology and Toxicology. Coordinator of the Masters Program from the Corporation of Basic Biomedical Sciences. Faculty of Medicine, Universidad de Antioquia, Medellín, Colombia. e-mail: sparra@quimbaya.udea.edu.co
6. Assistant Professor, Department of Pharmacology and Toxicology, Faculty of Medicine, Universidad de Antioquia. Coordinator of the Graduate Program in Clinical Toxicology, Universidad de Antioquia, Medellín, Colombia. e-mail: lpena@medicina.udea.edu.co
7. Bacteriologist, Laboratory of Toxicology, Department of Pharmacology and Toxicology, Faculty of Medicine, Universidad de Antioquia, Medellín, Colombia. e-mail: bmontoya@medicina.udea.edu.co
8. Associate Investigator, Group of Bio-pharmaceutical Research and Study, Universidad de Antioquia, Medellín, Colombia. e-mail: jriost@yahoo.com
9. Auxiliary Professor, Department of Pharmacy, Faculty of Pharmaceutical Chemistry, Universidad de Antioquia, Medellín, Colombia. e-mail: vitopa@farmacia.udea.edu.co
10. Assistant Professor, Department of Pharmacy, Faculty of Pharmaceutical Chemistry, Universidad de Antioquia. Coordinator of the Group of Bio-pharmaceutical Research and Study, Medellín, Colombia. e-mail: geib@farmacia.udea.edu.co
Received for publication December 17, 2009 Accepted for publication September 28, 2010
SUMMARY
Purpose: The aim of this study was to compare the bioavailability of two formulations of metformin 850 mg tablets: Glucophage® from Merck Santè laboratories (reference product) and Metformin from Winthrop Pharmaceuticals de Colombia SA (test product) in healthy Colombian volunteers.
Methods: A random, double blind, two-period, two-week wash out period, crossover study was performed in 24 healthy male and female volunteers for a single 850-mg dose of metformin tablets administrated with 240 ml of water after 12 hours of fasting. Once the drug was administrated, blood samples were collected before and within 24 hour, and plasma metformin concentration was determined by using a validated HPLC method. Pharmacokinetic parameters such as Cmax, AUC0-96h, AUC0-∞, and Tmax were determined. The formulations were considered bioequivalent if the logarithmic mean ratios of ln-transformed Cmax and AUC0-∞ values were within the equivalence range of 80%-125%.
Results: ANOVA analysis of the ln-transformed Cmax and AUC0-∞ indicated that none of the effects examined (formulation, period, within and between-subjet variances and carry over) was statistically significant. The mean (±SD) of Cmax 1217.38 (± 251.72) ng/ml vs. 1305.25 (± 301.06) ng/ml, AUC0-96h 1363.49 (± 315.51) ng.h/ml vs. 1584.82 (± 368.75) ng.h/ml, AUC0-∞, 7155.75 (± 1440.74) ng.h/ml vs. 7777.08 (± 1896.49) ng.h/ml, and Tmax 2.57 (± 0.93) h vs. 2.22 (± 0.94) h were obtained with test and reference formulations, respectively. These pharmacokinetic parameters presented differences with the results from other published papers. The 90% confidence interval of the logarithmic ratio of AUC0-∞ and Cmax was within the range of 80-125%.
Conclusions: In this study in healthy Colombian volunteers, a single 850-mg dose of metformin tablet test formulation met the criteria for bioequivalence to the reference formulation based on pharmacokinetic parameters AUC0-∞ and Cmax.
Keywords: Metformin; Bioequivalence; Bioavailability; Pharmacokinetics; Interchange of drugs; Area under curve.
Colomb Med. 2011; 42: 81-87
Comparación de la biodisponibilidad y la farmacocinética entre dos formulaciones de tabletas de metformina de 850 mg en voluntarios colombianos sanos
RESUMEN
Objetivo: El objetivo de este estudio es comparar la bioequivalencia de dos formulaciones de tabletas de metformina de 850 mg: Glucophage® del Laboratorio Merck Santè (producto de referencia) y metformina de Laboratorios Winthrop Pharmaceuticals de Colombia SA (producto de prueba), en voluntarios colombianos sanos.
Métodos: Se realizó un estudio aleatorizado, doble ciego, cruzado, en dos períodos y con un tiempo de lavado de dos semanas, en 24 voluntarios sanos, hombres y mujeres, que recibieron una dosis única de metformina de 850 mg, con 240 ml de agua, después de 12 horas de ayuno. Luego de la administración del medicamento, se recolectaron muestras de sangre durante 24 horas y las concentraciones plasmáticas de metformina se determinaron con un método de HPLC validado. Se calcularon los parámetros farmacocinéticos: Cmax, AUC0-96h, AUC0-∞, y Tmax. Las formulaciones se consideraron bioequivalentes si la relación de la media transformada a ln de Cmax y AUC0-∞ estaba dentro del rango de bioequivalencia de 80% a 125%.
Resultados: El Anova de los datos transformados a ln de Cmax y AUC0-∞ indicaron que ninguno de los efectos analizados (formulación, período, variación intra e intersujetos y arrastre) fueron estadísticamente significativos. La media (±SD) de los parámetros obtenidos para los productos de prueba y de referencia, respectivamente, fueron: Cmax 1217.38 (± 251.72) ng/ml vs. 1305.25 (± 301.06) ng/ml, AUC0-96h 1363.49 (± 315.51) ng.h/ml vs. 1584.82 (± 368.75) ng.h/ml, AUC0-∞, 7155.75 (± 1440.74) ng.h/ml vs. 7777.08 (± 1896.49) ng.h/ml, and Tmax 2.57 (± 0.93) h vs. 2.22 (± 0.94) h. El intervalo de confianza de la relación logarítmica del AUC0-∞ y Cmax se encontró dentro del rango de 80% a 125%.
Conclusiones: En este estudio en voluntarios sanos colombianos, la comparación de una formulación de prueba de tabletas de metformina de 850 mg, con una formulación de referencia, cumplió los criterios de bioequivalencia teniendo como base los parámetros farmacocinéticos AUC0-∞ and Cmax.
Palabras clave: Metformina; Bioequivalencia; Biodisponibilidad; Farmacocinética; Intercambiabilidad de medicamentos; Área bajo la curva.
Colomb Med. 2011; 42: 81-87
Metformin is a biguanide drug that became commercially available in 1957. It is for oral administration and has a specific anti-hyperglycemic effect on patients with type 2 diabetes mellitus (DM). Therapeutic doses of metformin do not produce hypoglycemia, and it is a therapeutic advantage when compared with sulfonylureas1.
Doses of 0.5-1.5 g have a bioavailability of 50%-60%2. Absorption is slow and incomplete in the upper gastrointestinal tract, because of the high polarity and low liposolubility of the molecule. At intestinal pH between 7 and 8, metformin is mainly ionized (pka=2.8 and 11.5), which slows its absorption rate3.
Metformin is rapidly distributed after absorption, and it is accumulated in the esophagus, stomach, duodenum, salivary glands, and kidneys4. It has neither binding to plasma proteins nor metabolism, and it undergoes renal excretion.
Nowadays, metformin is a first-choice drug for type 2 DM treatment because of its broad therapeutic advantages. Therefore, Colombian pharmaceutical industries have been motivated to produce a generic form of this drug.
Because of the low bioavailability and high inter-individual variability in the absorption of the different pharmaceutical forms of metformin, it is necessary to perform comparative bioavailability studies. Thus, regulatory authorities and medical prescribers would have the scientific support to expect a therapeutic equivalence if bioequivalence among the compared pharmaceutical forms is demonstrated.
The aim of this study was to evaluate, in healthy Colombian volunteers, the bioequivalence of the generic metformin, from Laboratorios Lakor Farmacéutica SA now Winthrop Pharmaceuticals de Colombia SA manufactured by Sanofi~Synthelabo, Cali, Colombia, with the reference product Glucophage® from Merck Santè laboratories.
MATERIALS AND METHODS
Drug products. Test product (T): metformin, 850-mg tablets, owned by Winthrop Pharmaceuticals de Colombia SA manufactured by Sanofi~Synthelabo, Cali, Colombia, lot: P1320804, and containing 850 mg of the active ingredient per tablet, corresponding to 100% of the labeled quantity of metformin.
Reference product (R): Glucophage®, 850 mg tablets, owned by Merck, manufactured by Merck Santè, Lyon, France, lot: 104031, and containing 872.95 mg of the active ingredient per tablet, corresponding to the 102.7% of the labeled quantity of metformin.
The pharmaceutical products used in this study were previously evaluated to determine the drug content of each product, according to the British Pharmacopoeia Quality Specifications. They were declared pharmaceutical equivalents because the test product did not differ from the reference product by more than 5%.
Study subjects. The study was conducted according to the Helsinki Declaration and Resolution 8430 of 1993 by the Ministry of Social Protection. It was also approved by the Ethics Committee of the School of Medicine, at Universidad de Antioquia. 24 subjects were recruited for this study, (10 male, 14 female; ages 21.2±2.1 years; weight 60.5±7.7 kg; height 1.67±0.08 m). Subjects were assessed healthy volunteers, after having been medically examined and clinically tested: complete blood count, urinalysis, blood biochemistry were normal, and HIV, hepatitis B, and pregnancy screenings (for women) were negative. All subjects were briefed on the bioequivalence study details and they all agreed and signed a written informed consent. All volunteers were free to leave the study at any time. The number of subjects was determined by the coefficient of variation from published data5 and by applying the method proposed by Zapater6 and Julious7 to obtain 80% power.
Clinical trial design. In order to evaluate the bioequivalence of the two metformin formulations, we performed a random, double blind, two-period crossover study. On the first dosing day, each subject took a metformin tablet of either test or reference formulations (850 mg of metformin) with 240 ml of water. After a 2-week wash out period, each subject took a tablet of the other product.
The volunteers for this study were admitted to the Corporación de Estudios en Salud clinic (CES) in Medellín (Colombia), and they were under direct medical supervision during 24 hours. The first day at 6:00 am, their urine samples were tested for alcohol and drug abuse. Subjects received formulations after 12 hours of fasting.
Standard breakfast, lunch, refection, and dinner were given at 2, 5, 8, and 11 hours after dosing. All meals were the same for both periods. Food with xanthines (such as chocolate), and carbonated drinks were not allowed.
Ten milliliter blood samples were taken through an indwelling cannula in heparinized tubes (Becton Dickinson, NY) before (0.0 h) and at 0.50, 1, 1.50, 2, 2.50, 3, 3.50, 4, 5, 6, 8, 10, 12, 14, and 24 hours after drug administration. Samples were centrifuged at 2000 rpm for 15 minutes and plasma was separated and stored at -20°C for analysis.
Analytical procedure and method validation. Metformin extraction from plasma was accomplished by the liquid-liquid extraction method proposed by Yuen et al.8 One half ml of plasma was vortex during 30 seconds in a screw-capped glass tube after adding 2 ml of acetonitrile to precipitate plasma proteins. After centrifugation (2500 rpm) for 5 minutes at 5ºC, 2 ml of supernatant was transferred to another clean glass tube. The drug was extracted with 2 ml of the extraction solvent (n-hexan) and vortex for thirty seconds followed by centrifugation (2800 rpm) for 5 minutes. The organic phase was then transferred by aspiration to a clean glass tube. The extraction procedure was repeated with the remaining samples. A gentle air flow and a water bath were used to dry the organic phase. The residue was reconstituted in 400 µl of a mixture of KH2PO4 buffer 10 mM (pH 7.5) and acetonitrile (72:28, v/v), and filtered by using a vacuum pump. One hundred microlitres of the sample were injected directly into a chromatographic system.
The HPLC system is comprised of an Agilent, model HP1100 (California, USA), pump, an Agilent diode array detector, and an auto-injector. The software package ChemStation (2000 Version) was used to control the chromatographic system. The Analytical column was a LiChrospher C18 RP-Select B (Agilent, 250 mm, 4 mm ID, 5 µm particle size). The mobile phase consisted of dihydrogen phosphate buffer 0.01M (pH 7.5) and acetonitrile (40:60 v/v). The flow rate was 1.5 ml/min.
The method was validated following criteria established by FDA Guidelines9. Calibration was linear in the concentration range of 62.5-2000.0 ng/ml, with an intra-day correlation coefficient of 0.9995 and inter-day of 0.9976. The intra-day and inter-day calibration curve showed consistent linearity, as seen by a slope coefficient of variation (CV) of 7.6% and 9.6%, respectively.
Other validation parameters were also fulfilled by this method. Intra-day precision was determined by replicate analysis (six times) of standard samples in plasma containing 125, 500, and 2000 ng/ml. Intra-day precision in this study, expressed as means of percent of CV, was 7.3%.
Inter-day precision was determined at six concentrations (62.5, 125, 250, 500, 1000, and 2000 ng/ml) in plasma, in six replicate runs (6 days). Inter-day precision in this study expressed, as the mean of CV was 12.3%. The limit of quantification based on CV smaller than 20% was 62.5 ng/ml; estimated on values obtained in intra-day and inter-day assays.
Two concentrations of metformin (250 and 1000 ng/ml-quality control (QC) samples in three replicates were used for stability studies, including freeze and thaw, short-term temperature and long-term stability; they fulfilled validation parameters. Standard curves were performed daily, over a 12-week period, with each volunteer’s plasma samples and showed consistent linearity (intercept, slope, and correlation coefficient).
Pharmacokinetic analysis. Pharmacokinetic data were calculated by non-compartmental method. The maximum plasma concentration (Cmax) and the time to reach it (Tmax) were determined by inspecting each individual plasma level-time curves. The elimination rate constant (ke) was obtained by ln-linear regression of the terminal decay phase. The area under the plasma level-time curve (AUC0-96h) was obtained by the trapezoidal rule, and the AUC96h-∞ time was determined by dividing the last plasma concentration by ke, and adding this result to the AUC0-96h. The partial AUC (as an early exposure measure) was obtained by truncating the partial area at the population median of Tmax values for the reference formulation.
Statistical analysis. In order to assess the effects of treatment, period, sequence of administration, and subjects, ln-transformed data for AUC0-∞ and Cmax, and non-transformed Tmax were evaluated by means of analysis of variance (ANOVA) for the cross design (Statistica 6.0, Statsoft Inc, 2001).
The method suggested by Schuirmann and accepted by the FDA10 (known as the two one-sided tests) was used to evaluate whether these two formulations of metformin were bioequivalent. Bioequivalence was accepted if 90% confidence intervals for test/reference ratios of AUCs and Cmax fell in the range of 0.80-1.25. A p value of less than 0.05 was considered statistically significant11.
RESULTS
At the first period, all 24 subjects concluded the study without any adverse effects. However, in the second period, one subject presented diarrhea with the test product, another reported epigastric pain with the reference product. At the beginning of this period, a subject was excluded because she was taking antibiotics to treat a urinary infection. Pharmacokinetic parameters were calculated for all 23 volunteers who ended the second period.
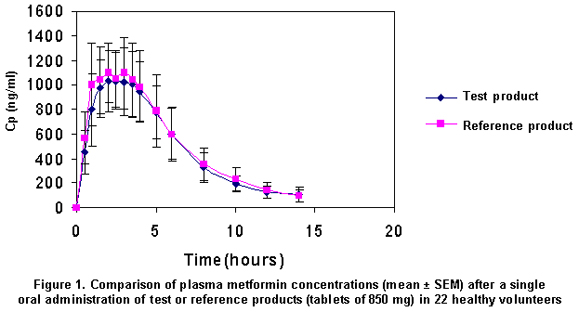
Concentrations over the quantification limit (62.5 ng/ml) were observed within 5 to 14 hours. Although blood samples were taken until 24 hours after drug administration, plasma levels were not detected at this time. The mean concentration profiles for the 2 formulations were quite similar, as observed in Figure 1.
To obtain paired data for 22 subjects, one of 23 was excluded randomly. All parameters had normality for intra-subject and inter-subject residues. However, two volunteers presented extreme outcomes: one had lower AUC0-∞ and early exposure area (AUC0-Tmax) values for the test as compared with the reference formulation; Tmax was also very different in this volunteer (test 5 hours, and reference 1.5 hours). The other one had higher AUC0-Tmax values for the test as compared with the reference formulation. The pharmacokinetic parameters for both formulations are shown in Table 1.
The mean values and 90% CI for the pharmacokinetic parameters compared are summarized in Table 2. ANOVA for AUC0-∞ and Cmax, after logarithmic transformation of the data, revealed that none of the effects examined (formulation, period, within and between-subject variances and carry over) was statistically significant. The 90% CI was within the bioequivalence acceptable range from 80% to 125%, suggesting that both formulations are similar.
DISCUSSION
Although we planned a study with 24 volunteers, we had to exclude two of them; nevertheless, the power test calculated by using the variability data obtained in the ANOVA analysis and the equations proposed by Julious7 revealed that a sample size of 22 volunteers was sufficient to reject bio-inequivalence (power 88.3%).
The available pharmacokinetic data on metformin have shown that oral bioavailability, ranges from approximately 32% to 61%. The drug disposition exhibits multi-compartmental characteristics. Metformin is rapidly eliminated by the kidney by combined glomerular filtration and tubular secretion. Its metabolism and protein-binding in plasma are negligible. Studies with small numbers of patients suggest that bioavailability decreases with increasing dose12. There are only scarce data on the relationship between plasma metformin concentrations and metabolic effects13.
Pharmacokinetic parameters such as ke, t1/2, and Cmax, present large differences when several papers are compared5,14,15 including the results obtained in this study and in another research we conducted16. This variability could be explained by the metformin accumulation in the intestinal wall17, the subject variation attributed to the drug transporter polymorphisms18, and physiological factors, such as gastric emptying and small-intestine transit. Changes in the gastric emptying and intestinal transit of a metformin dosage form may alter absorption processes and, therefore, bioavailability19.
The reference product reached slightly higher plasmatic levels than the test product and the amount absorbed seemed to be slightly greater (Figure 1). Although there was no significant difference for Cmax and AUC0-∞, the Tmax parameter showed a higher inter-subject variability, with one subject being the most extreme case, who showed a Tmax for the test product of 5 hours, while for the reference product it was 1.5 hours. For this reason, the partial AUC0-Tmax was measured.
The variability in Tmax had implications on the AUC0-Tmax, the 90% CI calculated for this parameter was 78.9-93.5 %, out the range of 80-125%. We might explain this result by the drug class, because in the biopharmaceutical classification it belongs to a substance with high solubility and low permeability (Class III)20. In a solution, the permeability may be the limiting factor for the absorption rate. Thus, the absorption kinetics should be ruled more by physio-logical conditions and the drug distribution than by factors related with the formulation21.
The differences found in this study for the Tmax can be demonstrated because it was performed with one dose only; however, with the chronic administration and the achievement of a stable concentration, no clinical repercussions are expected for such difference.
Although several parameters are important at the time of a product interchange, according to the FDA, the extension of the absorption (AUC0-∞), and the Cmax are the key parameters to declare the bioequivalence.
CONCLUSION
The generic product metformin, tablets of 850 mg, is bioequivalent regarding AUC0-∞ and Cmax when compared with the reference product (Glucophage®).
Conflict of interest. None of the authors has conflicts of interest related to this study.
ACKNOWLEDGMENTS
This study was sponsored by Winthrop Pharmaceuticals de Colombia SA, contract 8716-002 2003. We thank Professor Abel Díaz for his suggestions and advice with the statistical analysis, and the Pharmaceutical Chemistry students Andrés Restrepo and Iván Darío Gómez for their collaboration when the clinical trial was performed. We disclose no conflict of interest with the companies Winthrop Pharmaceuticals de Colombia S.A., Sanofi-Aventis or Merck-La Santé.
REFERENCES
1. Dunn CJ, Peters DJ. Metformin. A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs. 1995; 49: 721-49.
2. Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996; 30: 359-71.
3. Pentikainen PJ. Bioavailability of metformin. Comparison of solution, rapidly dissolving tablet and three sustained release products. Int J Clin Pharmacol Ther Toxicol. 1986; 24: 213-20.
4. Hale TW, Kristensen LP, Hackett LP, Kohan R, Ilett KF. Transfer of metformin into human milk. Diabaetologia. 2002; 1509-14.
5. Najib N, Idkaidek N, Beshtawi M, Bader M, Admour I, Alam SM, et al. Bioequivalence evaluation of two brands of metformin 500 mg tablets (dialon1 & glucophage1) in healthy human volunteers. Biopharm Drug Dispos. 2002; 23: 301-6.
6. Zapater P, Horga JF. Bioequivalencia y genéricos. Los estudios de bioequivalencia. I Una aproximación a sus bases teóricas, diseño y realización. Rev Neurol. 1999; 29: 1235-46.
7. Julious S. Tutorial in biostatistics. Sample sizes for clinical trials with normal data. Stat Med. 2004; 23: 1921-86.
8. Yuen KH, Peh KK. Simple high-performance liquid chromatographic method for the determination of metformin in human plasma. J Chromatogr B Biomed Appl. 1998; 710: 243-46.
9. Food and Drug Administration (FDA). 2001. Guidance for Industry. Bioanalytical Method Validation. (Accessed 25 Nov 2009). Available: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf.
10. Food and Drug Administration (FDA). 2001. Guidance for Industry - Statistical Approaches to Establishing Bioequivalence. (Accessed 25 Nov 2009). Available: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070244.pdf.
11. Food and Drug Administration (FDA) 2003. Guidance for industry, bioavailability and bioequivalence. Studies for orally administered drug products-general considerations. (Accessed 24 Nov 2009). Available: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070124.pdf.
12. Sambol NC, Chiang J, O'Conner M, Liu CY, Lin ET, Goodman AM, et al. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Pharmacol. 1996; 36: 1012-21.
13. Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996; 30: 359-71.
14. Atanasova I, Bozhinova K, Todorova D, Terziivanov D. Pharmacokinetics and comparative bioavailability of two metformin formulations after single-dose administration in healthy subjects. Clin Drug Invest. 2003; 23: 743-9.
15. Vlahov V, Thyroff-Friesinger U, Koytchev R, Bakracheva N, Gatchev E. Bioequivalence studies with metformin: comparability of reference tablets from different origins. Int J Clin Pharmac Ther. 2005; 43: 457-62.
16. Cuesta F, Holguín G, Archbold R, Restrepo M, Peña L, Montoya B. et al. Bioequivalencia de dos formulaciones de metformina, tabletas con 850 mg, en voluntarios sanos colombianos. IATREIA. 2005; 18: 289-301.
17. Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996; 334: 574-9.
18. Pacanowski MA, Hopley CW, Aquilante CL. Inter-individual variability in oral antidiabetic drug disposition and response: the role of drug transporter polymorphisms. Expert Opin Drug Metabolism Toxicol. 2008; 4: 529-44.
19. Marathe P, Wen Y, Norton J, et al. Effect of altered gastric emptying and gastrointestinal motility on metformin absorption. Br J Clin Pharmacol. 2000; 50: 325-32.
20. Cheng CL, Yu LX, Lee HL, Yang CY, Lue CS, Chou CH. Biowaiver extension to BCS class III high solubility-low permeability drugs: bridging evidence for metformin immediate-release tablet. Eur J Pharm Sci. 2004; 22: 297-304.
21. Yuen KH, Wong JW, Billa N, Julianto T, Toh WT. Bioequivalence of a generic metformin tablet preparation. Int J Clin Pharmac Ther. 1999; 37: 319-22.