Leishmania (Viannia) panamensis expresses a nuclease with molecular and biochemical features similar to the Endonuclease G of higher eukaryotes
Miguel A. Toro-Londoño, MSc1, Edwin Bairon Patiño, PhD2, Sara María Robledo, PhD3, Antonio Jiménez-Ruiz, PhD4, Juan Fernando Alzate, PhD5
1. Researcher, Program for the Study and Control of Tropical Diseases
(PECET), Medical School, Universidad de Antioquia, Medellín,
Colombia. e-mail: miguel.toro@yahoo.com
2. Professor, Institute of Chemistry, Universidad de Antioquia, Medellín, Colombia. e-mail: epatino_99@yahoo.com
3. Professor, Program for the Study and Control of Tropical Diseases
(PECET), Medical School, Universidad de Antioquia, Medellín,
Colombia. e-mail: sara_robledo@yahoo.com
4. Professor, Biochemistry and Molecular Biology Departament,
Universidad de Alcalá, Madrid, Spain. e-mail: antonio.jimenez@uah.es
5. Professor, Parasitology Group, Microbiology and Parasitology
Department, Medical School, Universidad de Antioquia, Medellín,
Colombia. e-mail: juan.alzate@medicina.udea.edu.co
Received for publication March 11, 2010 Accepted for publication June 30, 2010
SUMMARY
Objective: To characterize the molecular and biochemical features of the Endonuclease G of Leishmania (Viannia) panamensis.
Methods: The gene of the putative L. (V.) panamensis Endonuclease G was amplified, cloned, and sequenced. The recombinant protein was produced in a heterologous expression system and biochemical assays were run to determine its ion, temperature, and pH preferences.
Results: The L. (V.) panamensis rENDOG has biochemical features similar to those found in other trypanosomatids and higher eukaryotes. In addition, phylogenetic analysis revealed a possible evolutionary relationship with metazoan ENDOG.
Conclusions: L. (V.) panamensis has a gene that codifies an ENDOG homologous to those of higher organisms. This enzyme can be produced in Escherichia coli and is able to degrade covalently closed circular double-stranded DNA. It has a magnesium preference, can be inhibited by potassium, and is able to function within a wide temperature and pH range.
Keywords: Leishmania panamensis; Endonuclease G; Molecular characterization; Recombinant proteins; Refolding; Phylogeny.
Colomb Med. 2011; 42: 154-65
Leishmania (Viannia) panamensis expresa una nucleasa molecular y bioquímicamente similar a la Endonucleasa G de eucariotas superiores
RESUMEN
Objetivo: Caracterizar molecular y bioquímicamente la Endonucleasa G (EndoG) de Leishmania (Viannia) panamensis.
Métodos: El gen de la putativa Endonucleasa G de L. (V.) panamensis fue amplificado, clonado y secuenciado. La proteína recombinante se produjo en un sistema de expresión heterólogo y la proteína activa se sometió a pruebas bioquímicas para determinar la preferencia de iones, temperatura y pH.
Resultados: La rEndoG de L. (V.) panamensis muestra características bioquímicas similares a aquellas descritas en otros trypanosomatidos y en eucariotas superiores. Además, los análisis filogenéticos muestran una posible relación evolutiva con la Endonucleasa G de metazoos.
Conclusiones: Leishmania (V.) panamensis posee un gen que codifica para una endonucleasa homóloga a la EndoG de otros organismos superiores, que se puede producir de forma recombinante en Escherichia coli y que es capaz de degradar ADN circular cerrado de doble cadena. Tiene una preferencia por los iones magnesio y manganeso para usarlos como cofactor y es inhibida por el potasio. Además, funciona en un amplio rango de pH y temperatura.
Palabras clave: Leishmania panamensis; Endonucleasa G; Caracterización molecular; Proteínas recombinantes; Renaturalización; Filogenia.
Colomb Med. 2011; 42: 154-65
Leishmaniasis is a zoonosis caused by protozoan parasites of the Leishmania genus and transmitted by phlebotomine sand flies (Diptera: Psychodidae). This disease occurs in all continents except Antarctica and it is estimated that approximately 350 million people worldwide are at risk of infection with approximately 2.3 million new cases each year1. Leishmaniasis presents a wide clinical spectrum ranging from cutaneous forms that resolve spontaneously to visceral infections that may be fatal. The different clinical manifestations of the disease depend on the immune status of the host and the species of parasite involved. In Colombia, the most common clinical form is cutaneous leishmaniasis (CL) with 10,248 cases in 2008; Leishmania (Viannia) panamensis is the species most commonly implicated2.
Leishmania has a digenic life cycle, characterized by the presence of flagellate promastigotes in the insect vector and intracellular amastigotes within the phagosomes of host mammal macrophages. During the infection process in mammals, Leishmania parasites enter and remain within the host cell, evading the inflammatory response before causing the lesion3. It has been suggested that one of mechanisms by which the parasite evades the immune response is by phosphatidylserine (PS) expression in the cell membranes, both of promastigotes in the inoculum4 and amastigotes that simulate apoptosis5. After being recognized by phagocytic cells, this PS induces production of the anti-inflammatory cytokine TGB-β and causes negative regulation of the pro-inflammatory cytokine TNF-α6,7; thus, reducing inflammation and allowing the parasite to evade the immune system. Despite the importance of processes similar to apoptosis in these parasites, the mechanisms and molecules involved have been poorly studied. Recently, the presence of a mitochondrial nuclease, which migrates to the nucleus in response to apoptotic stimuli8,9 has been identified, both in species of Trypanosoma (T. brucei) and Leishmania, i.e., L. (Leishmania) donovani and L. (L.) infantum. This protein is thought to be homologous to the Endonuclease G (EndoG) of higher eukaryotes, responsible for DNA degradation in caspase-independent processes of programmed cell death10,11.
EndoG has been characterized in different organisms, both multi- and uni-cellular, and it is known for participating in apoptotic processes. EndoG also plays a role in the repair and replication of mitochondrial DNA12. This enzyme is a non-specific DNA/RNA nuclease and belongs to a family of nucleases denominated ββα-me finger, a name based on the disposition of the catalytic site, which comprises two beta sheets and an alpha helix13. This site is also characterized by the moiety DRGH, histidine (H) being responsible for the activation of the water molecule needed to carry out hydrolysis of the phosphodiester bond9,14. It should be noted that in trypanosomatids this characteristic moiety is replaced by SRGH8,9. The change from aspartic acid to serine does not affect enzyme activity because this site is not critical for catalytic activity14. Furthermore, its activity is known to be principally dependent on Mg+2, Mn+2, or Co+2, which distinguishes it from DNAses I and II12.
In this study we demonstrate for the first time that L. (V.) panamensis also possesses a gene that codes for this enzyme, which upon being expressed in a recombinant manner presents endonuclease activity under conditions known to be present during the apoptotic process. Additionally, a phylogenetic relationship between this protein in trypanosomatids and its presumed homologue in higher eukaryotes is established for the first time.
MATERIALS AND METHODS
Parasite culture and isolation of nucleic acids. Promastigotes of the L. (V.) panamensis strain MHOM/CO/87/UA140 were cultured in modified biphasic Novy-MacNeal-Nicolle (NNN) medium supplemented with penicillin (2000 U/ml). The cultures were initiated with an inoculum of 106 parasites/ml and the parasites harvested in the logarithmic or stationary phase after incubation at 26°C for 5 days. DNA was purified from these promastigotes using standard protocols with phenol/chloroform.
Amplification and cloning of the putative endog of L. (V.) panamensis. The primers 5EGbrcx (sense) 5' GGGGATCCGCCCAGGCCTCCACGCTCA 3' and 3EGbrcx (anti-sense) 5' GGCTCGAGCTGCCGGTA CCGCTGGAAGA 3', which contain recognition target for the restriction endonucleases BamHI and XhoI, respectively, were used to amplify the gene in L. (V.) panamensis. These primers were designed based on the internal region of the putative endog of L. (V.) braziliensis (LbrM10_V2.0750). The final concentrations were 1 mM of MgCl2, 200 nM of each primer, 200 mM of dNTP, 100 ng of genomic DNA, 2.5 U/100µl of Taq polymerase and 5% DMSO. The amplification conditions were: 1 cycle for 5 min at 95ºC, 30 cycles each for 45 s at 95ºC, 30 s at 55ºC, 2 min at 72ºC, and finally one 7-min cycle at 72ºC. PCR products were analyzed by electrophoresis in 1% agarose and stained with ethidium bromide with an expected product of 1077 bp. The amplified band and the expression vector pET28 a(+) (Novagen, Germany) were digested by using the restriction endonucleases BamHI and XhoI (Fermentas, Lithuania) and separated by electrophoresis in 1% agarose. The bands corresponding to the gene and the digested vector were cut from the gel and purified by using the Illustra GFXTM commercial kit (GE Healthcare, UK). The purified products were bound with the enzyme T4 DNA ligase (Fermentas, Lithuania) to create the construct denominated pET28a (+) - endog. This construct was used to transform competent bacteria DH5a (Invitrogen, USA) by thermal shock. These were plated out on LB plates with 50 µg/ml kanamycin as a selection antibiotic. Four colonies were selected from the bacteria that grew on the plate and grown in liquid LB with antibiotic. The plasmid was purified by using the QIAprep Spin Miniprep commercial kit (QIAGEN, Germany), and sequenced on both strands to confirm the content of the insert (Macrogen, South Korea).
Expression and purification of the rENDOG of L. (V.) panamensis. The pET28 a(+)-endog construct was used to transform competent BL21 (DE3) bacteria (Stratagene, USA) by thermal shock. These were plated out in LB plates with 50 µg/ml kanamycin as a selection antibiotic. A preinoculum in liquid LB with antibiotic was prepared from these transformed bacteria, grown for 16 h and then diluted 1:100 in 500 ml of fresh medium to carry out the induction. Induction was performed when the culture reached an optical density (OD) of 0.6 at 600 nm, isopropyl b-D-1-thiogalactopyranoside (IPTG) being added over 2 h to produce a final 1-mM concentration. The induction was separated by electrophoresis in polyacrylamide gel with 12% SDS (SDS-PAGE) and stained with Coomassie blue for an expected 43 kDa band, corresponding to the rENDOG of L. (V.) panamensis. Solubility of the protein was determined according to the protocol found in QIAexpressionist (QIAGEN, Germany). Because the expression vector pET28 a(+) adds a double tail of histidines (his-tag) to the aminoterminal and carboxyterminal regions of the protein of interest, purification was performed by means of affinity chromatography with columns of nickel-agarose (Ni-NTA) (QIAGEN, Germany). This was performed under denaturing conditions, always using the same buffer (100 mM NaH2PO4, 10 mM Tris·Cl, 8 M urea) and changing the pH only during lysis (pH 8.0), washings (pH 6.3), and elutions (pH 4.9 and pH 4.5), according to the protocol described in QIAexpressionist. The fractions collected were separated by 12% SDS-PAGE and stained with Coomassie blue.
Refolding and quantification of the partial rENDOG of L. (V.) panamensis. Given that purification was carried out under denaturing conditions to obtain active protein, the elution fractions had to be refolded by dilution. This was performed by diluting the concentration of the chaotropic agent, in this case urea, to allow the protein to fold and adopt a more stable three-dimensional configuration. This was perfomed by adding buffer without urea gradually every hour, reducing the urea concentration in the final volume by 0.5 units each time until a 0.25-M concentration was reached.
Quantification was performed after the refolding process by using the Bradford method (Sigma-Aldrich, USA). Five µl was added to each of the BSA standards at a range of 0.1-1.4 mg/ml BSA in different wells, the same being performed with the problem protein. Subsequently, 250 µl of Bradford reagent was added to each well and agitated for 30 s before being incubated for 30 s in darkness at room temperature. Absorbance of the standards was read at 595 nm and plotted against the concentration on a graph. Concentration of the control sample was determined from the straight-line equation obtained. The assays were carried out in triplicate.
Tests of nuclease activity. To evaluate the nuclease activity of the refolded protein, a test digestion was performed using 1 µg rENDOG and 1 µg of covalently closed circular DNA with the buffer TangoTM 1x (Fermentas©) for 2 h at room temperature and 37°C. To determine the optimal concentration of magnesium, digestions were carried out by using 1 µg of recombinant enzyme, 1 µg of covalently closed circular DNA and a buffer suitable for endonucleases containing: 10 mM Tris·Cl at pH 7.5, 10 mM DDT, 1 mg/ml BSA and 50 mM NaCl at 37°C for 2 h. The effect of different mono- and divalent cations on nuclease activity was evaluated in the same way. Increasing concentrations of 1, 5, 10, and 20 mM of the cations barium (Ba2+), calcium (Ca2+), and manganese (Mn2+) were evaluated. The inhibitory concentration of potassium was determined in the same manner by using the optimal determined concentration of magnesium.
Effects of pH and temperature on enzymatic activity. The effects on enzymatic activity of pH over a range of 3-12 and temperature at 20-84°C were determined under the conditions mentioned previously and the obtained optimal concentration of magnesium.
Bioinformatical analysis. Bioinformatic analyses such as the search for homologous sequences to construct the phylogenetic tree were performed by using the ExPASy database (http://ca.expasy.org/). The search for the signal peptide was performed on the amino acid sequence of L. (V) braziliensis using the SP-NN algorithm in the TriTrypDB database (http://tritrypdb.org/tritrypdb/). This same database was used to determine the position of the gene on the genomes of the different species of trypanosomatids (synteny).
Functional domains were sought in the protein expressed by using the conserved domains database (CCD) tool from the NCBI (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). Extremity sequences belonging to L. (V.) braziliensis were used to determine the relevance of the amino and carboxyterminal ends absent in the rENDOG of L. (V.) panamensis. Secondary structures (alpha helix and beta sheets) in these sequences that could give indications of their importance were sought by using the APSSP2 algorithm (http://www.imtech.res.in/raghava/apssp2/).
Phylogenetic relationship. To make a phylogenetic reconstruction of the Endo G of L. (V.) panamensis, 26 sequences of proteins homologous to human Endo G were identified from a wide variety of organisms, including bacteria, fungi, amphibians, birds, and mammals. The sequences collected were aligned using the Clustal algorithm and the BLOSUM matrix. To determine the evolutionary relationship of the 26 taxa analyzed, the alignment data were loaded onto the MEGA 4 program and a distance matrix obtained that showed the estimated divergence between the sequences. These results were obtained from pairwise analysis between the 26 alignments using Poisson's correction method. Based on the sequence alignment data obtained, a tree was constructed by the Neighbour-Joining (NJ) method to determine evolutionary relationships among the 26 organisms of the gene studied. The bootstrap used was 1000 and those branches reproduced in less than 50% of the replicates were collapsed.
RESULTS
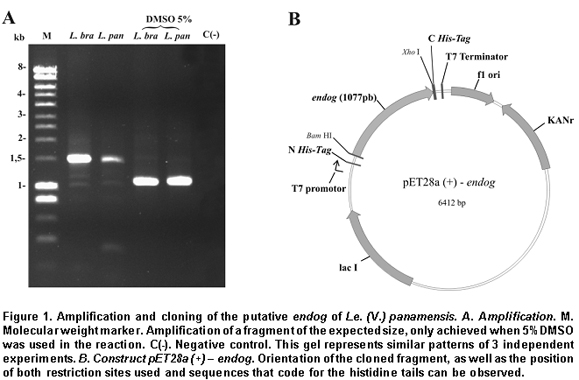
Amplification and cloning of the putative endog of L. (V.) panamensis. A 1077 bp internal region of the putative endog gene of L. (V.) panamensis was amplified by PCR. This amplification was performed after adding 5% DMSO to the PCR mix, given that without this additive a non-specific band of approximately 1500bp (Figure 1A) was amplified. This region was cloned into the plasmid pET 28 (+), creating the construct pET28a (+) - endog (Figure 1B). The construct insert was sequenced for both strands and aligned against the putative endog gene of L. (V.) braziliensis using the Clustal W algorithm, yielding a 98% identity as a result. This way, it was confirmed that the amplified and cloned fragment was the internal region of the putative endog gene of L. (V.) panamensis. The sequence obtained can be found by using GeneBank access number GQ119624.1.
Expression and purification of the rENDOG of L. (V.) panamensis. Based on its nucleotide sequence, the protein deduced was of 401 amino acids with a molecular mass of 43.3 kDa. After induction with 1 mM IPTG for 2 h at 37°C, bacteria BL21 (DE3) transformed with pET28a (+)-endog expresses a protein of approximately 45 kDa, corresponding to the expected size of the rENDOG, was observed (Figure 2A). Solubility studies of the recombinant protein revealed that this was expressed in an insoluble form (data not shown), hence purification had to take place under denaturing conditions (Figure 2B). After purifying 0.96 g of a bacterial precipitate, 65 mg of total soluble protein was obtained and then, 10.5 mg of purified recombinant protein was obtained from the elutions. Since the Bradford methodology is incompatible with urea concentrations above 3 M, these fractions were quantified by spectrophotometry at 280 nm.
Refolding and quantification of the partial rENDOG of L. (V.) panamensis. After refolding, a final 30-ml volume was obtained with a protein concentration of 100 ng/ml. Quantification was performed via the Bradford methodology. Given that during the refolding process by dialysis, as well as dilution of the chaotropic agent the protein was also diluted, precipitation with trichloroacetic acid (TCA) was carried out before quantification. When samples were treated with 400 mM dithiothreitol (DTT) the bands of highest molecular weight observed in purified fractions of rENDOG disappeared (Figure 3A).
Nuclease activity assay. The preliminary test of nuclease activity in the buffer TangoTM 1x, Fermentas©, showed the protein obtained from the refolding process to be active both at room temperature and at 37°C. Its endonuclease nature was clear from its degradation of covalently closed circular DNA (Figure 3B). Enzymatic activity was shown by the appearance of a DNA smear, a clear sign of degradation of this macromolecule.
Optimal concentration of MgCl2 and inhibitory concentration of KCl. After testing the preliminary activity, a nuclease activity assay was performed with MgCl2. This revealed that the optimal concentration of Mg2+ for 1µg of rENDOG is 15 mM, no further increase of nuclease activity being seen above this concentration (Figure 4A). Furthermore, it was determined that the inhibitory concentration of K1+ is 140 mM. A gradual reduction in nuclease activity was observed as potassium concentation increased, total inhibition was observed at 230 mM (Figure 4B).
Effect of different divalent cations on nuclease activity. Because the enzyme uses divalent cations present in the cellular environment as cofactors, the activity was evaluated by using divalent cations other than magnesium such as: barium, manganese, and calcium. Despite being a divalent cation, barium does not function as a cofactor (Figure 5A). Manganese does behave as a cofactor, albeit less effectively than magnesium (Figure 5C). For calcium, concentrations of 1-5 mM presented endonuclease activity (Figure 5B); although, much less than that observed with magnesium and manganese, indicating that it is a less efficient cofactor.
Effects of pH and temperature on enzymatic activity. It was found that the enzyme maintained its nuclease activity over a wide pH range of 3-12 (Figure 6A). It also worked over a wide range of temperatures, remaining active from 20-50°C (Figure 6B). In similar experiments performed in our laboratory (results not shown), we demonstrated that the enzyme was able to maintain its activity for up to 2 h at 84°C, inactivation occurring after exposure to 100°C for more than 5 min.
Bioinformatic analysis. Bioinformatic analysis revealed that EndoG possesses a signal peptide of approximately 32 amino acids on the aminoterminal end. It was also determined that the gene coding for EndoG is located on chromosome 10 in the three Leishmania species for which the genome is completely known i.e., L. (L.) infantum, L. (L.) major, and L. (V.) braziliensis. It was also determined that this is a highly syntenic single-copy gene in these species. The enzyme was found to possess three known domains, one denominated NUC referring to a super family of nucleases, one binding to magnesium, and a third binding to the substrate (DNA). In the absent aminoterminal region, it was determined that the missing amino acids did not form any secondary structure that could indicate a biological function. The algorithm predicted the formation of an alpha helix on the carboxyterminal region with high probability (>0.8), although no known functional domain was identified.
Phylogenetic relationship. Sequences were aligned based on the amino acid sequence obtained for the Endo G of L. (V.) panamensis (Figure 7A), as well as on the other 26 protein sequences presumably homologous to this. A phylogenetic tree was constructed from this alignment by the Neighbour Joining (NJ) method in which the phylogenetic relationship that exists between the protein of L. (V.) panamensis and the other known homologues is shown (Figure 7B).
DISCUSSION
Although various studies have been carried out on Trypanosoma and Leishmania to show the existence of ENDOG4,8,9, all Leishmania studies were performed on species of the Leishmania subgenus and their relevance to members of Viannia species is unknown. It has been established that certain genes that exist in one subgenus are lacking in the other and vice-versa15; hence, it is important to carry out independent studies on Viannia species. Certain differences were clear from the start of the study given that it was not possible to amplify the entire gene in L. (V.) panamensis using oligonucleotides designed from sequences of the endog of L. (L.) major, L. (L.) infantum, and L. (V.) braziliensis (results not shown). Thus, we may conclude that the 5' and 3' terminal sequences of the coding region (CDS) of endog differ from those of the reference species mentioned and for this reason did not adequately hybridize the oligonucleotides in amplification PCR of the entire gene. For this reason, new oligonucleotides were designed based on the internal region of the gene of L. (V.) braziliensis, allowing amplification of a band of approximately 1500 bp, which however did not concur with the expected size of the internal 1077-bp region we attempted to amplify. By using 5% DMSO as an additive, it was posible to amplify and clone a 1077-bp fragment of the internal coding region of the Endo G gene of L. (V.) panamensis. It should be noted that although it is an internal region of the gene, it contains the triplet codons of the amino acids included in the putative catalytic site of the enzyme. This fragment corresponds to 71% of the expected size of the gene, based on the 1515-bp sequence reported for this gene in L. (V.) braziliensis (LbrM10_V2.0750). In lacking the 5' end of the coding region, we know that 86 amino acids are missing from the aminoterminal end of the enzyme of which the first 40 correspond to the signal peptide that naturally directs this enzyme to the mitochondria of the parasite9. Our in-silico analysis revealed the presence of a signal peptide of 32 amino acids on the amino terminal. According to the predictions performed, the other missing amino acids do not form any secondary structure and, thus, cannot be performing any function. Likewise, by missing part of the 3' region, the enzyme lacks 58 amino acids in the carboxy-terminal region, which according to the predictions has a very high probability of forming a pair of alpha helix. This indicates that the region may code for some biological function; nevertheless, more studies are rendered. Despite these deletions in the amino and carboxyterminal ends, the rENDOG of L. (V.) panamensis is an active endonuclease able to degrade covalently closed circular DNA, demonstrating that these regions are not critical to the functioning of this enzyme. This is to be expected given that in Leishmania this enzyme is 53 kDa, much larger than the 32 kDa found in other eukaryotic organisms such as humans and fungi, as well as the 37 kDa of the nuclease of Serratia marcescens, which is also thought to be related to the ENDOG of higher organisms10,12.
The high expression levels obtained for the rENDOG of L. (V.) panamensis in E. coli facilitated the formation of inclusion bodies16, requiring a denaturation process of solubilization of the protein for purification. The high amount of protein produced during expression is surprising, given that in some species such as L. (L.) major, the toxicity of the enzyme precludes it from being produced in recombinant manner in E. coli8. Although L. (L.) infantum ENDOG has been produced in E. coli, its levels of expression are not as high9. It, therefore, remains to be determined whether the high levels seen in L. (V.) panamensis are due to the lower toxicity of recombinant proteins from New World species per se, or if the absence of the signal peptide and/or the carboxy-terminal region reduces their toxicity, allowing better expression to occur.
On carrying out rENDOG purification, high molecular weight proteins were found as contaminants in the elution fractions. However, these disappeared when the quantity of DDT (dithiothreitol) in the buffer of the protein sample was duplicated to 400 mM, indicating that they were actually multimeric aggregates of the recombinant protein bound to each other by disulphate bridges. This enzyme is known to require a divalent cation as a cofactor, thought to be involved in stabilization of the transition state during nucleophilic attack and in coordination of the water molecule that protonates the salient group during hydrolysis14. Among the ions known to function as cofactors in orthologous enzymes are cobalt (Co2+), manganese (Mn2+), magnesium (Mg2+), nickel (Ni2+), calcium (Ca2+), and zinc (Zn2+)12. Even though the enzyme is able to function with several of the ions mentioned, it is widely accepted that the most important is Mg2+. It is known that the ENDOG of C. elegans, which has been implicated in apoptosis, is dependent only on magnesium11. Given the importance of Mg2+ as the principal cofactor of ENDOG, a test was run to determine the optimal concentration of this cation. It was thus determined that the optimal concentration for 1 µg of recombinant enzyme is 15 mM of MgCl2, without increase in enzymatic activity above this concentration. However, a concentration of 50 mM of MgCl2 appeared to inhibit activity. Rather than being due to excess cofactor, this inhibitory effect could be explained by the effect of magnesium on DNA because the magnesium can bind to the major and minor grooves of the DNA molecule, altering the DNA structure17. It is noteworthy that the intracellular concentration of magnesium dissolved in the cytosol lies between 0.5 and 1 mM18, concentration at which rENDOG is active. Different mono- and divalent cations, such as barium (Ba2+), manganese (Mn2+), calcium (Ca2+), and potassium (K1+) were evaluated in addition to magnesium. As expected, both manganese and calcium function as cofactors, clear signs of degradation were visible after 2 h of digestion. Although greater signs of degradation were observed with magnesium and manganese than with calcium, the importance of the latter in the activation of nuclease activity during apoptosis has been demonstrated in mammals19,20. However, no degradation whatsoever was evident for barium, perhaps due to its greater size than other divalent cations. This would cause a steric impediment, which would not permit the enzyme to use the cation as a cofactor. After determining the optimal magnesium concentration for the enzyme, an inhibition assay was performed by using increasing concentrations of potassium. It was determined that concentrations above 140 mM of KCl inhibited enzymatic activity, with complete inhibition at 230 mM. These results concur with the intracellular potassium concentration of 140 mM both in mammalian cells and Leishmania promastigotes. The potassium concentration, nevertheless, falls to 30 mM in mammalian cells and 65 mM in promastigotes during apoptosis21-23. It is clear that under normal intracellular conditions, the enzyme is slighly inhibited due to the intracellular concentrations of potassium. Because Leishmania is a parasite with a heteroxenous life cycle, exposed to different temperatures both in the insect vector and in the mammalian host, we decided to evaluate the temperature range at which ENDOG mantains its activity and determined that this enzyme is active above 20°C, presenting peak activity at 40-46°C. Enzymatic activity began to be affected above 48°C, with residual nuclease activity observed up to 84°C, which means that this enzyme is highly thermostable. No major changes were observed in its degradation capacity when activity was evaluated at pH levels of 3-12. The changes observed in electrophoretic migration can be attributed to the high concentrations of NaOH used to reach the most alkaline pH values (unpublished laboratory observations). It is not surprising that the enzyme is tolerant to a wide pH range because it is known that the pH tends to acidify during apoptotic processes.
The homologous relationships among the endonucleases G of distinct organisms have been established mainly based on similarity of enzyme functions than on phylogenetic analysis. For this reason, 26 protein sequences presumably homologous to human ENDOG were collected and a phylogenetic tree was constructed to determine the evolutionary relationships among these proteins. ENDOG belongs to the super family of non-specific nucleases denominated ββα-Me-finger, although the catalytic moiety DRGH present in most members of this family14 is modified in trypanosomatids since a serine residue occupies the position of the aspartic acid (SRGH). Although it has been suggested that this aspartic acid acts on conformation of the catalytic site, mutagenesis site assays suggest that the change from aspartic acid to alanine produces a 54% reduction in bovine ENDOG activity. The function of aspartic acid in trypanosomatids appears to be performed without any problem by serine. The other residues critical to function, including the asparagine responsible for binding to the cofactor24, are present in the rENDOG of L. (V.) panamensis. Even with this change in the sequence, it aligned adequately with the other 25 sequences analyzed and did not require mayor editing. Based on this alignment, a distance matrix was constructed from this tree by the Neighbour-Joining method. Proteins belonging to trypanosomatids are grouped together in this tree, as are those from bacteria, fungi, insects, and mammals. From these results, it can be inferred that the putative ENDOG of these parasites is a homologue of the one found in humans and other higher eukaryotes. Thus, from the point of view of possible structure and function9 this similarity, together with the hypothesis of a possible common origin of this gene shown by the construction of this tree, strengthens the hypothesis for homology among these enzymes.
In summary, we may conclude that L. (V.) panamensis possesses a gene that codes for an endonuclease homologous to the ENDOG of higher organisms, which can be produced in recombinant form in E. coli and is able to degrade covalently closed circular double-stranded DNA. It has a preference for magnesium and manganese ions, using them as cofactors, and is inhibited by potassium. It also functions over a wide range of pH and temperature. The similarity in the biochemical characteristics and relationship of this enzyme with those found in metazoans, suggest that it could be involved in the process of programmed cell death that has been described for these organisms.
Conflict of interest. None of the authors has conflicts of interest related to this study.
ACKNOWLEDGEMENTS
This project received joint funding from the research support foundation at Banco de la República de Colombia (Project N° 2204), Universidad de Antioquia and COLCIENCIAS (Project N° 1101-00018-9999 and 1102-343-19319) and Centro para el Desarrollo de Productos (CIDEPRO).
REFERENCES
1. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004; 27: 305-18.
2. Vélez ID, Gilchrist K, Arbeláez MP, Rojas CA, Puerta JA, Antunes CM, et al. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Trans R Soc Trop Med Hyg. 2005. 99: 593-8.
3. Belkaid Y, Méndez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged «silent» phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000; 165: 969-77.
4. Zandbergen V, Bollinger GA, Wenzel A, Kamhawi S, Voll R, Klinger M, et al. Leishmania disease development depends on the presence of apoptotic promastigotes in the virulent inoculum. Proc Natl Acad Sci U S A. 2006; 103: 13837-42.
5. de Freitas JM, Moreira ME, Bonomo A, Bozza PT, Amarante G, Pirmez C, et al. Apoptotic mimicry by an obligate intracellular parasite downregulates macrophage microbicidal activity. Curr Biol. 2001; 11: 1870-3.
6. Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997; 390: 350-1.
7. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM, Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998; 101: 890-8.
8. Gannavaram S, Vedvyas C, Debrabant A, Conservation of the pro-apoptotic nuclease activity of endonuclease G in unicellular trypanosomatid parasites. J Cell Sci. 2008; 121: 99-109.
9. Rico E, Alzate JF, Arias AA, Moreno D, Clos J, Gago F, et al. Leishmania infantum expresses a mitochondrial nuclease homologous to EndoG that migrates to the nucleus in response to an apoptotic stimulus. Mol Biochem Parasitol. 2009; 163: 28-38.
10. Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001; 412: 95-9.
11. Parrish J, Li L, Klotz K, Ledwich D, Wang X, Xue D, Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature. 2001; 412: 90-4.
12. Low RL, Mitochondrial Endonuclease G function in apoptosis and mtDNA metabolism: a historical perspective. Mitochondrion. 2003; 2: 225-36.
13. Kuhlmann UC, Moore GR, James R, Kleanthous C, Hemmings AM. Structural parsimony in endonuclease active sites: should the number of homing endonuclease families be redefined? FEBS Lett. 1999; 463: 1-2.
14. Schafer P, Scholz SR, Gimadutdinow O, Cymerman IA, Bujnicki JM, Ruiz A, et al. Structural and functional characterization of mitochondrial EndoG, a sugar non-specific nuclease which plays an important role during apoptosis. J Mol Biol. 2004; 338: 217-28.
15. Peacock CS, Seeger K, Harris D, Murphy L, Ruiz JC, Quail MA, et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat Genet. 2007; 39: 839-47.
16. Marston FA. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J. 1986; 240: 1-12.
17. Jerkovic B, Bolton PH. Magnesium increases the curvature of duplex DNA that contains dA tracts. Biochemistry. 2001; 40: 9406-11.
18. Permyakov EA, Kretsinger RH. Cell signaling, beyond cytosolic calcium in eukaryotes. J Inorg Biochem. 2009; 103: 77-86.
19. Crawford DR, Abramova NE, Davies KJ. Oxidative stress causes a general, calcium-dependent degradation of mitochondrial polynucleotides. Free Radic Biol Med. 1998; 25: 1106-11.
20. Trump BF, Berezesky IK. Calcium-mediated cell injury and cell death. FASEB J. 1995; 9: 219-28.
21. Bortner CD, Hughes FMJr, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem. 1997; 272: 32436-42.
22. Hughes, FMJr, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem. 1997; 272: 30567-76.
23. Sen N, Das BB, Ganguly A, Mukherjee T, Bandyopadhyay S, Majumder HK. Camptothecin-induced imbalance in intracellular cation homeostasis regulates programmed cell death in unicellular hemoflagellate Leishmania donovani. J Biol Chem. 2004; 279: 52366-75.
24. Friedhoff P, Kolmes B, Gimadutdinow O, Wende W, Krause KL, Pingoud A. Analysis of the mechanism of the Serratia nuclease using site-directed mutagenesis. Nucleic Acids Res. 1996; 24: 2632-9.