The clinical utility of tuberculin skin tests: a single-center experience
Keywords:
Tuberculin Test, Interferon-gamma Release Tests, Latent Tuberculosis, BCG Vaccine, Tuberculin, Mycobacterium tuberculosisMain Article Content
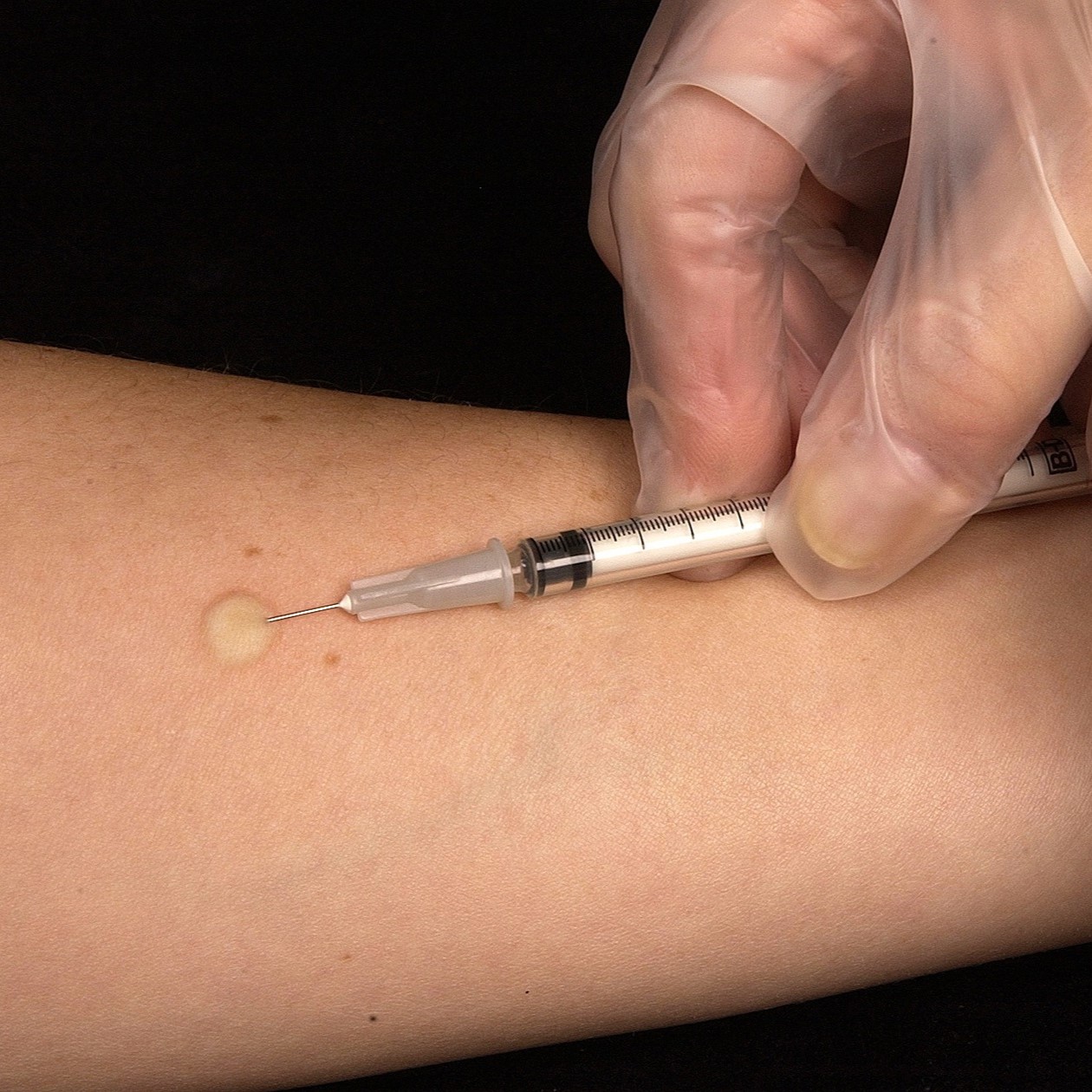
Background: Tuberculin skin test (TST) has an important place in the diagnosis of , latent tuberculosis infection (LTBI) for nearly a century
Objective: This study aimed to investigate the general characteristics of patients that were tested with TST in a university hospital within two years.
Methods: Patients that were tested with TST were included study. The Mantoux method was used for the administration of TST. All patients were assessed with regards to LTBI.
Results: A total of 661 patients, 345 (52.2%) men and 316 (47.8%) women, with a mean age of 43.0±15.9 years, were included in the study. Accordingly, TST was performed prior to anti-TNF biological agent therapy for 50% (331) of the participants, for LTBI screening before solid organ and/or hematological stem cell transplantation for 20.4% (135), for screening following contact with tuberculosis for 25.1% (166), for screening of healthcare professionals for 1.1% (7), and for medical report for 3.3% (22). 2.7% of the patients who took TST were diagnosed with active tuberculosis (14 with pulmonary tuberculosis and 4 with extrapulmonary tuberculosis). QuantiFERON-TB Gold (QFT) test was performed in 332 (50.2%) patients with anergic TST results. According to TST and QFT test results, 28.3% (187) of the patients were started on tuberculosis prophylaxis.
Conclusion: While TST is most commonly performed for LTBI screening prior to biological agent therapy, almost one fourth of patients taking TST require tuberculosis prophylaxis. About half of the patients, on the other hand, require an additional QFT test.
Global tuberculosis report 2020. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO. Accessed September 1, 2021. https://www.who.int/publications-detail-redirect/9789240013131
Türkiye’de Verem Savaşı 2020 Raporu, T.C. Sağlık Bakanlığı Halk Sağlığı Genel Müdürlüğü, Yayın no: 1205, Ankara, 2021. Accessed October 7, 2022. https://hsgm.saglik.gov.tr/tr/tuberkuloz-haberler/turkiye-de-verem-savasi.html
Özkara Ş, Kılıçaslan Z. Tüberküloz. AVES yayıncılık. Istanbul, 2010; 11:36-47
No authors listed. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161(4 Pt 2):S221-247. doi:10.1164/ajrccm.161.supplement_3.ats600 DOI: https://doi.org/10.1164/ajrccm.161.supplement_3.ats600
Zevallos K, Vergara KC, Vergara A, Vidal C, Garcia HH, Evans CA. Tuberculin Skin-Test Reactions Are Unaffected by the Severity of Hyperendemic Intestinal Helminth Infections and Co-Infections. Am J Trop Med Hyg. 2010; 83(2): 319-325. doi:10.4269/ajtmh.2010.10-0073 DOI: https://doi.org/10.4269/ajtmh.2010.10-0073
Gümüşlü F, Özkara Ş, Özkan S, Baykal F, Güllü Ü. Türkiyede verem Savaşı 2007 raporu. Verem Savaş Daire Başkanlığı Ankara 2007.
Tüberküloz tanı ve tedavi rehberi. T.C Sağlık Bakanlığı (Yayın no: 1129); Ankara, 2019.
Hanta I, Ozbek S, Kuleci S, Kocabas A. The evaluation of latent tuberculosis in rheumatologic diseases for anti-TNF therapy: experience with 192 patients. Clin Rheumatol. 2008;27(9):1083-1086. doi:10.1007/s10067-008-0867-3 DOI: https://doi.org/10.1007/s10067-008-0867-3
WHO. Latent tuberculosi sinfection (Executive summary). Updated and consolidated guidelines for programmatic management. Geneva. World Health Organization; 2018. Accessed September 3, 2021. Available from: https://www.who.int/tb/publications/2018/executivesummary_consolidated_guidelines_ltbi.pdf?ua=
Acar M, Sütçü M, Salman N, Somer A. The Risk of Tuberculosis and TNF-alpha Inhibitors. J Pediatr Inf 2017; 11(2): e71-e75. Doi: 10.5578/ced.201719. DOI: https://doi.org/10.5578/ced.201719
Anti-TNF Kullanan Hastalarda Tüberküloz Rehberi. T.C Sağlık Bakanlığı Türkiye Halk Sağlığı Kurumu.Ankara; 2016.
Pyo J, Cho SK, Kim D, Sung YK. Systemic review: agreement between the latent tuberculosis screening tests among patients with rheumatic diseases. Korean J Intern Med. 2018;33(6):1241-1251. doi:10.3904/kjim.2016.222 DOI: https://doi.org/10.3904/kjim.2016.222
Dogan C, Kiral N, Comert SS, Fidan A, Caglayan B, Salepci B. Tuberculosis Frequency in Patients Taking TNF-alpha Blokers. Tur Toraks Der. 2012;13(3):93-98. doi:10.5152/ttd.2012.22 DOI: https://doi.org/10.5152/ttd.2012.22
Manuel O, Humar A, Preiksaitis J, Doucette K, Shokoples S, Peleg AY, et al. Comparison of quantiferon-TB gold with tuberculin skin test for detecting latent tuberculosis infection prior to liver transplantation. Am J Transplant. 2007;7(12):2797-2801. doi:10.1111/j.1600-6143.2007.02011.x DOI: https://doi.org/10.1111/j.1600-6143.2007.02011.x
Malhotra KK, Dash SC, Dhawan IK, Bhuyan UN, Gupta A. Tuberculosis and renal transplantation--observations from an endemic area of tuberculosis. Postgrad Med J. 1986;62(727):359-362. doi:10.1136/pgmj.62.727.359 DOI: https://doi.org/10.1136/pgmj.62.727.359
Roth PJ, Grim SA, Gallitano S, Adams W, Clark NM, Layden JE. Serial testing for latent tuberculosis infection in transplant candidates: a retrospective review. Transpl Infect Dis. 2016;18(1):14-21. doi:10.1111/tid.12489 DOI: https://doi.org/10.1111/tid.12489
Muñoz P, Rodríguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis. 2005;40(4):581-587. doi:10.1086/427692 DOI: https://doi.org/10.1086/427692
Ahmadinejad Z, Azmoudeh AF, Razzaqi M, Davoudi S, Jafarian A. QuantiFERON-TB Gold In-Tube test for diagnosis of latent tuberculosis (TB) infection in solid organ transplant candidates: a single-center study in an area endemic for TB. Transpl Infect Dis. 2013; 15(1): 90-95. doi:10.1111/tid.12027 DOI: https://doi.org/10.1111/tid.12027
Horsburgh CR, Rubin EJ. Clinical practice. Latent tuberculosis infection in the United States. N Engl J Med. 2011; 364(15): 1441-1448. doi:10.1056/NEJMcp1005750 DOI: https://doi.org/10.1056/NEJMcp1005750
Barnes PF. Diagnosing latent tuberculosis infection: turning glitter to gold. Am J Respir Crit Care Med. 2004;170(1):5-6. doi:10.1164/rccm.2404004 DOI: https://doi.org/10.1164/rccm.2404004
Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
Lee JE, Kim HJ, Lee SW. The clinical utility of tuberculin skin test and interferon-γ release assay in the diagnosis of active tuberculosis among young adults: a prospective observational study. BMC Infect Dis. 2011; 11(96):1-7. doi:10.1186/1471-2334-11-96 DOI: https://doi.org/10.1186/1471-2334-11-96
Ozcirpici B, Aydin N, Coskun F, Tuzun H, Ozgur S. Vaccination coverage of children aged 12-23 months in Gaziantep, Turkey: comparative results of two studies carried out by lot quality technique: what changed after family medicine? BMC Public Health. 2014; 14: 217. doi:10.1186/1471-2458-14-217 DOI: https://doi.org/10.1186/1471-2458-14-217
Horsburgh CRJ. Priorities for the treatment of latent tuberculosis infection in the United. N Engl J Med 2004;350:2060-7. doi: 10.1056/NEJMsa031667 DOI: https://doi.org/10.1056/NEJMsa031667
Downloads

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The copy rights of the articles published in Colombia Médica belong to the Universidad del Valle. The contents of the articles that appear in the Journal are exclusively the responsibility of the authors and do not necessarily reflect the opinions of the Editorial Committee of the Journal. It is allowed to reproduce the material published in Colombia Médica without prior authorization for non-commercial use

 https://orcid.org/0000-0003-3800-9126
https://orcid.org/0000-0003-3800-9126